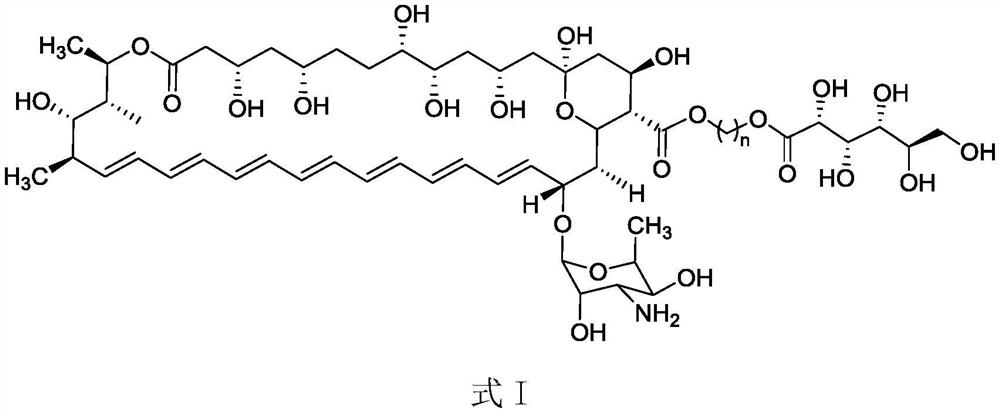
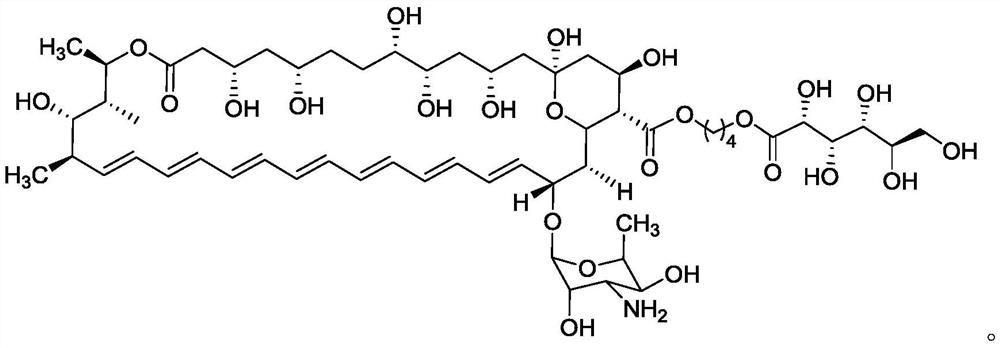
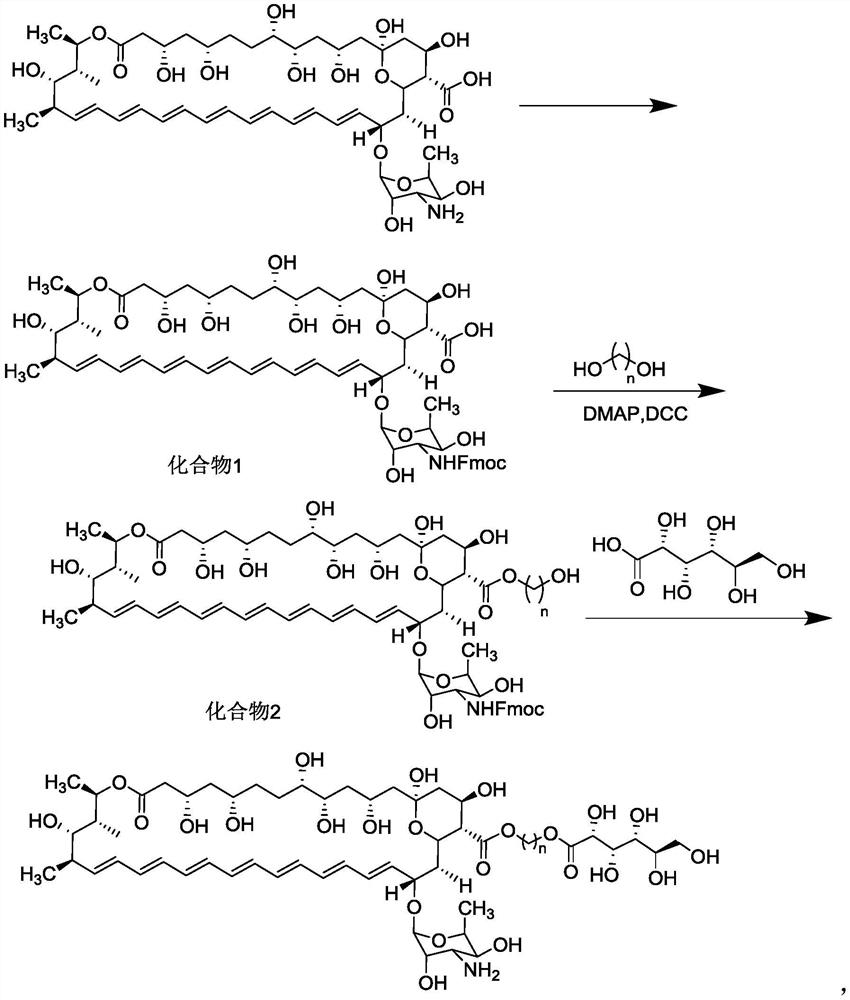
Ester derivative of amphotericin B and application of ester derivative
A technology for amphotericin and medicine, which is applied in the field of ester derivatives of amphotericin B and its use as an antifungal agent, and can solve the problems of poor water solubility of amphotericin B and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Weigh amphotericin B (1.033g, 1.12mmol), Fmoc-Osu (0.852g, 2.52mmol), add to a 250mL round bottom flask, use ultra-dry DMF (60mL) and methanol (30mL) as a mixed solvent , after stirring to dissolve, pyridine (99%, 0.85 mL) was added dropwise. Under nitrogen atmosphere, the reaction was carried out at room temperature and protected from light for 24 hours. The reaction progress was monitored by thin-layer chromatography. After the reaction was completed, most of the solvent was distilled off under reduced pressure. Then it was added dropwise into a cold diethyl ether (200 mL) solution, and a pale yellow solid was precipitated, and the crude product was collected by centrifugation or vacuum filtration. Purified by flash column chromatography to obtain 1.05g of compound 1, yield: 81.8%; m / z: 1145.5 [M-H + ].
[0044] 2) Dissolve compound 1 (575mg, 0.5mmol) and 1,4-butanediol (360mg, 4mmol) in 10mL of anhydrous THF, add DMAP (61.0mg, 0.5mmol) and DCC (390mg, 1.5mmol) ...
Embodiment 2
[0048] Example 2 In Vitro Activity Test
[0049] Antifungal activity in vitro was determined using a serial dilution method in 96-well microplates in buffered medium RPMI 1640 pH 7.0 according to standard methods (National Committee for Clinical Laboratory Standards). At wavelength λ=531nm (A 531 ), the optical density of the cell suspension was measured using a microplate reader. On the basis of the obtained results, a graph of the relationship between the A531 value and the concentration of the test compound was made. From these graphs, read the IC50 value, which is the interpolated concentration of the test compound at which A 531 The value is exactly the A of the control sample 531 50% of the value. In addition, the MIC value, which is the lowest concentration of the test compound at which A 531 The value is the measured A of the control sample 531 A maximum of 20% of the value.
[0050] Blood toxicity assays were performed by serial dilution according to known meth...
Embodiment 3
[0053] Example 3 Cytotoxicity Test to Mammalian Cells
[0054] Cell lines used for detection: CCRF-CEM-human acute lymphoblastic leukemia; HepG2-human malignant liver cancer, LLC-PK1-pig kidney epithelial cells; all cell lines were purchased from commercial sources.
[0055] Culture CCRF-CEM cells in RPMI 1640+10% fetal bovine serum (FBS) medium, culture LLC-PK1 cells in medium 199+3% FBS medium, culture HepG2 cells in MEM+10% FBS medium . All media contained 100 μg / ml of penicillin G and streptomycin. will be 1.2×10 4 An amount of cells / well of cells was seeded in a 24-well microplate containing an appropriate medium and allowed to stand overnight. Next, test compounds were added in a volume of 10 μl (serial 2x dilutions) as a solution in dimethyl sulfoxide (DMSO). Add 10 μl of DMSO to the control wells. The microplate with the cell suspension was incubated at a temperature of 37° C. for 120 hours in an atmosphere of 95% / 5% CO 2 . After incubation, add 200 μl of 3-(4,5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com