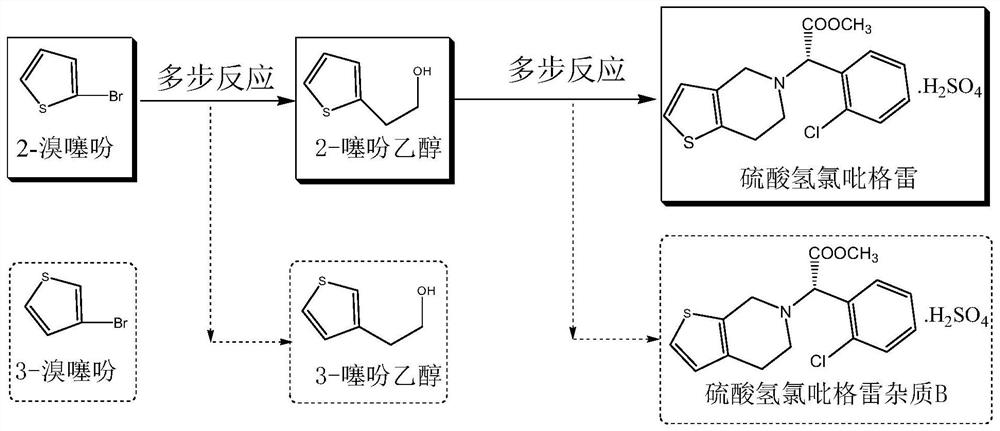
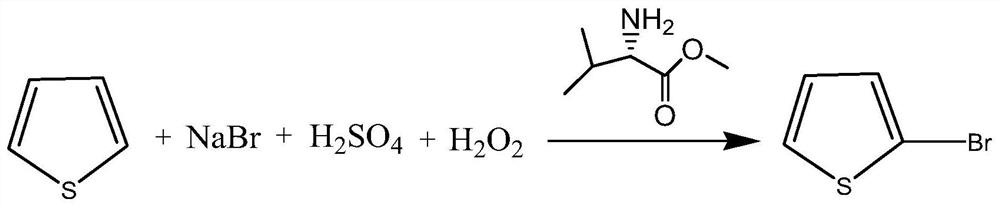Preparation method of 2-bromothiophene
A bromothiophene and thiophene technology is applied in the field of preparation of fine chemical products, and can solve the problems of not revealing the 2-position substitution selectivity of thiophene and reducing the generation amount of 3-bromothiophene.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
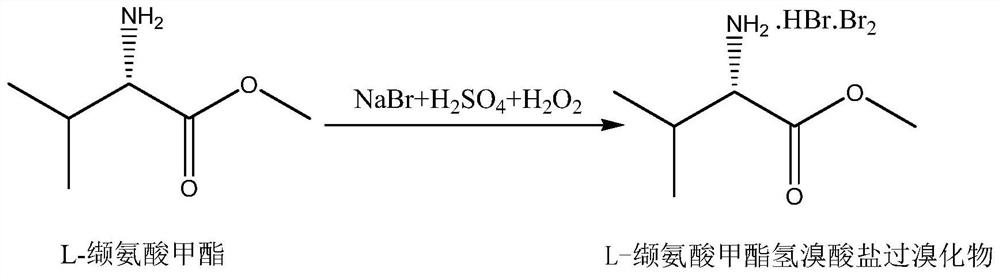
[0028] Add 52.8g (0.513mol) of commercially available sodium bromide, 7.4g (0.056mol) of commercially available L-valine methyl ester, and 340ml of water into a 500ml reaction bottle. Sulfuric acid 64.3g (0.642mol), after dropwise addition, cool down to 5-10°C, add 45.0g (0.535mol) of thiophene, then add 62.4g (0.642mol) of 35% hydrogen peroxide dropwise at 5-10°C, about 1-10°C 2 hours to drip. After dropping, control the temperature at 5-10°C for 18 hours, let stand, and separate layers. The organic layer was steam distilled to obtain 2-bromothiophene, wherein the content of 3-bromothiophene was 0.12%, see Figure 4 .
Embodiment 2
[0030] Drop into 400g of valsartan waste water in the 500ml reaction bottle, contain sodium bromide 52.8g (0.513mol), L-valine methyl ester 7.4g (0.056mol) in the waste water, control feed liquid temperature ≤ 40 ℃ under stirring, drop Add 64.3g (0.642mol) of concentrated sulfuric acid, after the dropwise addition, cool down to 5-10°C, add 45.0g (0.535mol) of thiophene, and then add 62.4g (0.642mol) of 35% hydrogen peroxide dropwise at 5-10°C, approx. 1 to 2 hours to drop. After dropping, control the temperature at 5-10°C for 18 hours, let stand, and separate layers. The organic layer was steam-distilled to obtain 2-bromothiophene, wherein the content of 3-bromothiophene was 0.14%, see Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com