Carboxy-DiI compound as well as preparation method and application thereof
A compound and product technology, applied in the field of detection, can solve the problems of inapplicable detection and evaluation, and achieve the effects of high fluorescent labeling efficiency, high specificity, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a synthetic method of Carboxy-DiI type compound A, comprising the following steps:
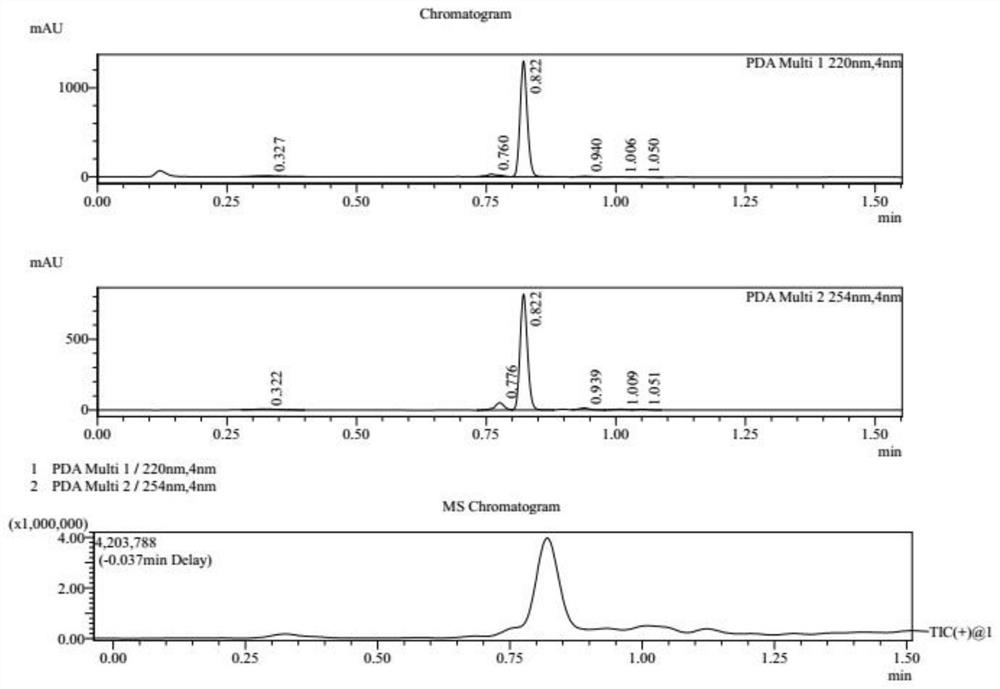
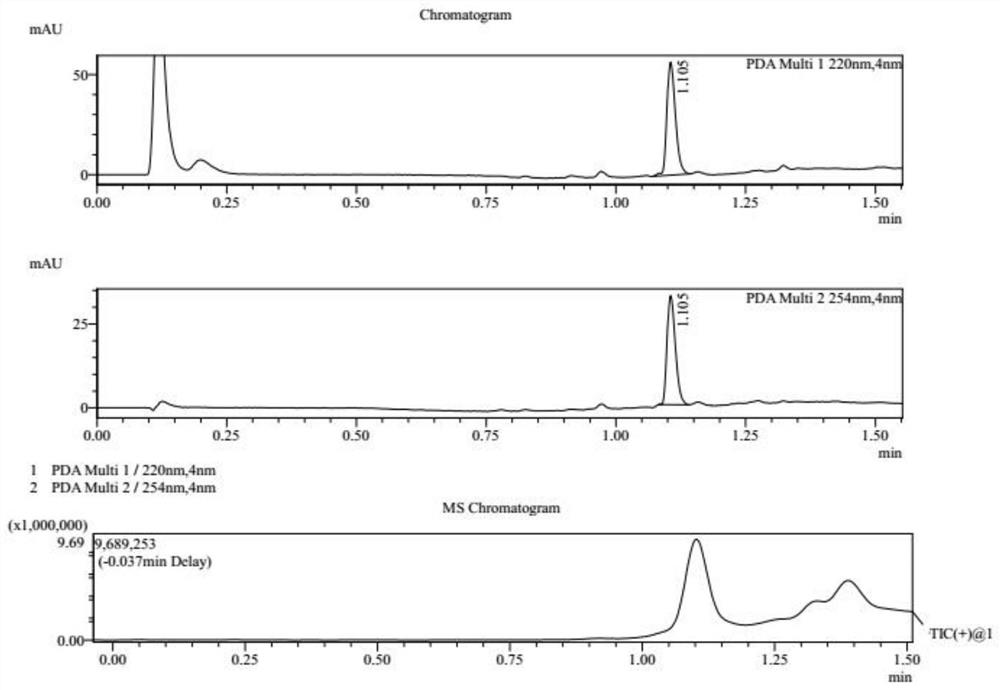
[0030] S1, referring to the following reaction formula, at 25°C, dissolve compound 1 (10.0g, 1.00eq, HCl) in 80mL acetic acid solution (containing compound 1a, 7.71g, 9.57mL, 2.00eq), and heat to 130°C , Reacted for 16h to obtain a reaction mixture containing compound 2. After washing the reaction mixture with 200 mL of water, the reaction mixture was extracted three times with ethyl acetate, each time 100 mL, to obtain an organic layer mixture, which was then washed with saturated NaHCO 3 Adjust the pH of the aqueous solution to 8, absorb water with anhydrous sodium sulfate, and dry in vacuo to obtain compound 2 as a brown oil with a yield of 88.6% and a purity of 94.4%. The reaction process of compound 2 is monitored by LCMS, and the results are as attached figure 1 shown.
[0031]
[0032] S2, referring to the following reaction formula, compound 2 (4.0...
Embodiment 2
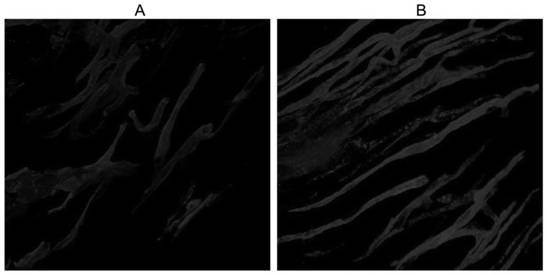
[0039] This embodiment provides a method for detecting arterial blood vessel distribution using Carboxy-DiI compound labeling, comprising the following steps:
[0040] (1) Heart perfusion, preparation of tissue samples:
[0041]The experimental rats were subjected to cardiac perfusion 45 minutes after ligation of the left anterior descending artery. Before the start of cardiac perfusion, connect 2 three-way stopcocks and 3 10mL syringes, which are respectively filled with PBS buffer solution and working solution containing Carboxy-DiI compounds (take 5 mg of Carboxy-DiI compound A prepared in Example 1) Dissolve the powder in 1mL of absolute ethanol, then transfer to dissolve in 50mL of buffer solution mixed with PBS buffer solution and 5% glucose solution at a volume ratio of 1:4) and 4% paraformaldehyde, and empty the air bubbles in the syringe . Use a 25-gauge butterfly needle to puncture and fix it from the left ventricle, open the right atrium and cut an opening to drai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com