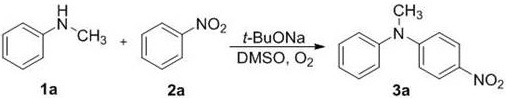
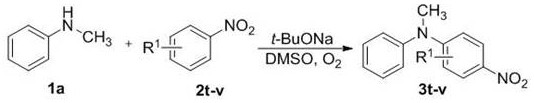
A sort of n -Preparation method of aryl-substituted heterocyclic compound
A technology of heterocyclic compounds and compounds, applied in the field of organic chemical synthesis, can solve the problems of long-term reaction under harsh conditions, heavy metal pollution, and difficult removal of guiding groups, etc., and achieve the effect of simple synthesis process, rapid reaction and excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of 3a-3h.
[0024] Will N -Methylanilines 1a-1h (0.5 mmol), t -BuONa (294 mg, 1.5 mmol), nitrobenzene 2a (184.5 mg, 1.5 mmol), added to 2.0 mL of dimethyl sulfoxide (DMSO), reacted at room temperature for 60 minutes to stop the reaction, quenched with water, dichloro Methane extraction, column chromatography separation (using silica gel column; eluent: petroleum ether / ethyl acetate = 20 / 1), to obtain pure product N -Methyldiphenyl derivatives 3a-3h.
[0025] Its synthetic route is as follows:
[0026]
[0027] N -methyl-4-nitro- N -phenylaniline: 3a
[0028]
[0029] Yellow solid, the yield is 83%; 1 H NMR (400 MHz, CDCl 3 ) δ 7.98 -7.92 (m, 2H), 7.36(t, J = 7.8 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H), 7.16 -7.11 (m, 2H), 6.57 (dd, J = 7.3, 5.3 Hz, 2H), 3.31 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 153.7, 146.3, 138.0, 130.2, 126.7, 126.6, 125.7, 112.3, 40.5.
[0030]4-fluoro- N -methyl- N -(4-nitrophenyl) aniline: 3b
[0031] ...
Embodiment 2
[0051] Example 2: Preparation of 3i, 3j.
[0052] Will N -Ethylanilines 1i, 1j (0.5 mmol), t -BuONa (294 mg, 1.5 mmol), nitrobenzene 2a (184.5 mg, 1.5 mmol), added to 2.0 mL of dimethyl sulfoxide (DMSO), reacted at room temperature for 60 minutes to stop the reaction, quenched with water, dichloro Methane extraction, column chromatography separation (using silica gel column; eluent: petroleum ether / ethyl acetate = 20 / 1), to obtain pure product N -Methyldiphenyl derivatives 3i, 3j.
[0053] Its synthetic route is as follows:
[0054]
[0055] N -ethyl-4-nitro- N -phenylaniline: 3i
[0056] Yellow solid, yield 71%; 1 H NMR (400 MHz, CDCl 3 ) δ8.03 (d, J = 9.4 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.34 (t, J = 7.4 Hz, 1H),7.26-7.15 (m, 2H), 6.64-6.55 (m, 2H), 3.82 (q, J = 7.1 Hz, 2H), 1.27 (t, J =7.1 Hz, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 153.1, 144.9, 137.8, 130.3, 127.8, 127.1, 126.0, 112.3, 47.2, 12.3.
[0057]N -ethyl-4-methyl- N -(4-nitrophenyl)anilin...
Embodiment 3
[0059] Example 3: Preparation of 3k, 3l.
[0060] Will N, N - diphenylamine compound 1k, 1l (0.5 mmol), t -BuONa (294 mg, 1.5 mmol), nitrobenzene 2a (184.5 mg, 1.5 mmol), added to 2.0 mL of dimethyl sulfoxide (DMSO), reacted at room temperature for 60 minutes to stop the reaction, quenched with water, dichloro Methane extraction, column chromatography separation (using silica gel column; eluent: petroleum ether / ethyl acetate=20 / 1), to obtain pure product N -Methyldiphenyl derivatives 3k, 3l.
[0061] Its synthetic route is as follows:
[0062]
[0063] 4-nitro- N,N -diphenylaniline: 3k
[0064]
[0065] Yellow solid, the yield is 67%; 1 H NMR (400 MHz, CDCl 3 ) δ 8.05 (t, J = 6.3 Hz, 2H), 7.39 (t, J = 7.8 Hz, 4H), 7.28 -7.18 (m, 6H), 6.94 (t, J = 6.3 Hz, 2H). 13 CNMR (100 MHz, CDCl 3 ) δ 153.6, 145.8, 140.3, 130.1, 126.7, 125.9, 125.6, 118.3.
[0066] 3-methyl- N -(4-nitrophenyl)- N -phenylaniline: 3l
[0067]
[0068] Yellow solid, yield 42%; 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com