Electrode for electrochemical reduction of carbon dioxide and application thereof
A carbon dioxide, electrochemical technology, applied in electrodes, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problems of unclear selectivity and active mechanism, and achieve smooth products/reactions The effect of mass transfer channel, improving selectivity, and promoting diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] An electrode for electrochemical reduction of carbon dioxide and its application, including a catalyst, the catalyst is an ordered core-shell catalyst, and the catalyst is Cu-vacancy copper / Cu 2 O, where Cu-vacancy copper is the shell, and Cu 2 O is the nucleus, and the composition of the catalyst is: Cu 50-90wt%, vacancy copper 5-30wt%, Cu 2 O 5~20wt%, the preparation method of described carbon dioxide electrochemical reduction electrode comprises:
[0032] S1: Clean the copper mesh, nickel foam and copper foil base layer with water, ethanol or acetone, and then wash them in H 3 PO 4 and leveling at a constant potential voltage of 3V to 5V for 5s to 1200s, then washed and dried for later use;
[0033] S2: Immerse the base layer in a mixture of a certain amount of sodium hydroxide and an oxidizing agent, the oxidizing agent is one or two of ammonium persulfate, potassium persulfate, sodium persulfate, hydrogen peroxide, and potassium dichromate, And the concentratio...
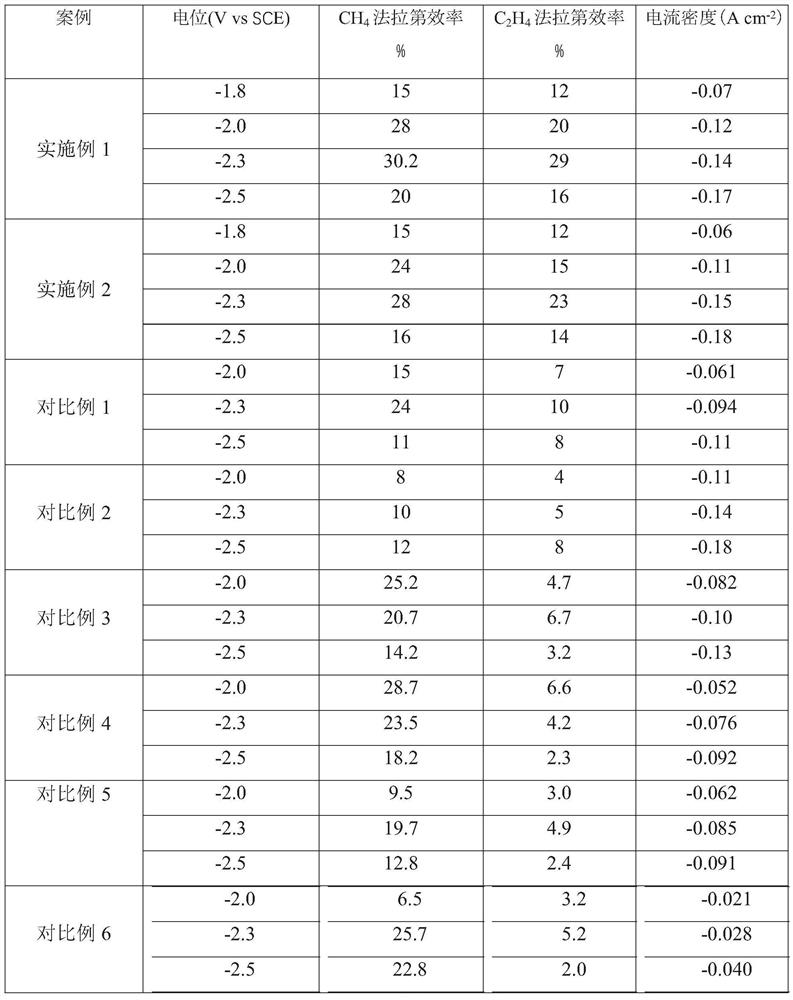
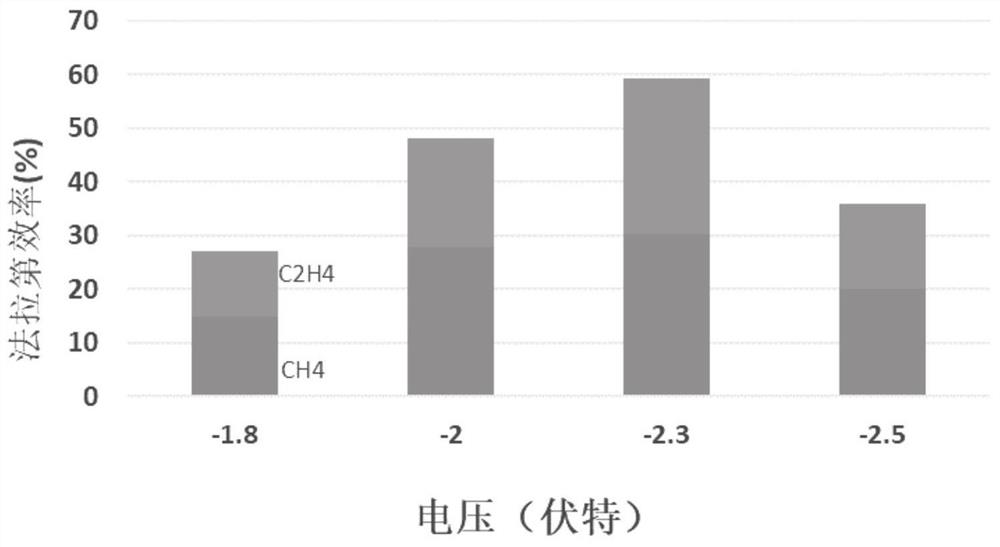
Embodiment 1
[0039] With the copper grid as the base, ultrasonically wash in ultrapure water and ethanol for 10 min, and then wash in 95% H 3 PO 4 In the process, leveling was carried out for 900s under the condition of a constant potential voltage of 4V, washed and dried for later use; the base layer was immersed in a mixture of 2M aqueous sodium hydroxide solution and 0.05M ammonium persulfate, and the ratio of sodium hydroxide to oxidant was 15: 1. Continue to react for 30 minutes and dry at 100°C to obtain CuO x Nanowire / Cu base layer; with the molar concentration ratio of disodium edetate dihydrate and Cu in copper salt as 1:1, 0.4M CuCl 2 2H 2 O mixed with disodium edetate dihydrate and treated with H 2 SO 4 Adjust the pH of the copper salt solution to 1.0 as the electrolyte, using a deposition current density of -150mAcm -2 , deposited for 60s, and then with a deposition current density of -60mAcm -2 , deposited for 1200s, as attached figure 1 As shown, after cleaning, place ...
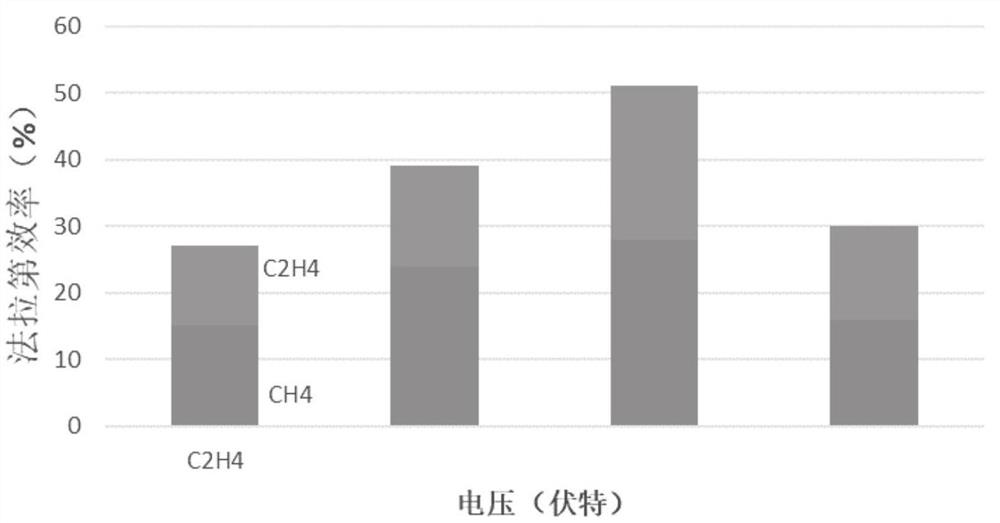
Embodiment 2
[0041] With the copper mesh as the base, ultrasonically wash in ultrapure water and ethanol for 10 min, and then wash in 95% H 3 PO 4 In the process, the voltage of the constant potential is 4V, and the leveling is carried out for 900s, washed and dried for later use; the base layer is immersed in a mixture of 1.5M sodium hydroxide aqueous solution and 0.05M potassium persulfate, and the ratio of sodium hydroxide and oxidizing agent is 10 : 1, continue the reaction for 40min, and dry at 100°C to obtain CuO x Nanowire / Cu base layer; the molar concentration ratio of disodium ethylenediaminetetraacetic acid dihydrate and Cu in copper salt was 1:1, and 0.2M Cu(NO 3 ) 2 ·3H 2 O mixed with disodium edetate dihydrate and treated with H 2 SO 4 Adjust the pH of the copper salt solution to 1.0 as the electrolyte, using a deposition current density of -120mAcm -2 , deposited for 80s, and then with a deposition current density of -60mAcm -2 , deposited for 1500s, as attached figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com