Triazole active molecule-based photoaffinity probe molecule as well as preparation method and application thereof
A technology of active molecules and probe molecules, which is applied in the field of photoaffinity probe molecules based on triazole active molecules and its preparation and application, can solve the problem of probe molecules that cannot be stably combined with drug target molecules and the activity of probe molecules Reduction, poor cell permeability and other problems, to achieve the effect of improving the inability to stably bind drugs and the decrease in activity of probe molecules, simple preparation method, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
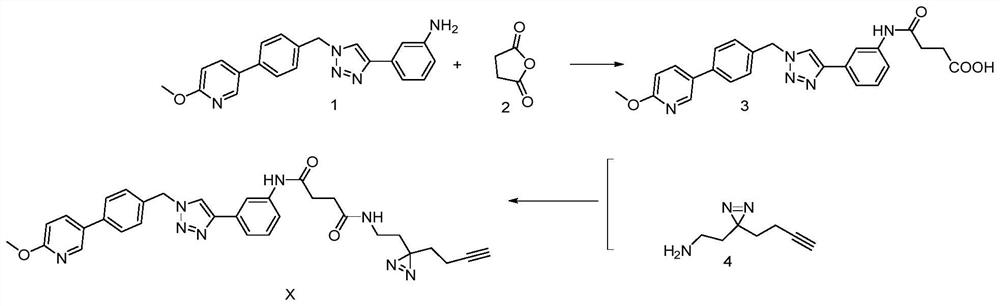
[0032] see figure 1 , a method for preparing a photoaffinity probe molecule based on a triazole active molecule, comprising the following steps:
[0033] 1) 3-(1-(4-(6-methoxypyridin-3-yl)benzyl)-1H-1,2,3-triazol-4-yl)aniline (i.e. triazole active molecule) Reaction with succinic anhydride in acetonitrile solution to obtain an intermediate product with a monocarboxylic acid;
[0034] The specific operation of the step 1) is: dissolve 1.40mmol of triazole active molecule and 2.10mmol of succinic anhydride in 10mL of acetonitrile solution, react at 60°C for 8h, after the reaction is completed, spin off the organic solvent under low pressure, add an appropriate amount of water, Extracted with ethyl acetate, the extracted organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a crude product, which was separated by a chromatographic column to obtain an intermediate product with monocarbox...
Embodiment 1
[0042] see figure 1 , the structural formula of the photoaffinity probe molecule based on the triazole active molecule, which has the function of target confirmation, is prepared by the following steps:
[0043] Dissolve triazole active molecules and succinic anhydride in acetonitrile, react at 60°C for 8 hours to obtain an intermediate product with monocarboxylic acid; the specific process is as follows:
[0044] Dissolve 1.40mmol of triazole active molecule and 2.10mmol of succinic anhydride in 10mL of acetonitrile solution, react at 60°C for 8h, after the reaction is completed; spin off the organic solvent under low pressure, add an appropriate amount of water, extract with ethyl acetate, and extract the organic phase After washing with saturated brine and drying over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product, which was separated by chromatography and eluted with petroleum ether / ethyl acetate (V / V=1 / 1) to obtain th...
Embodiment 2
[0051] Application of photoaffinity probe molecules based on triazole active molecules in the confirmation of target proteins.
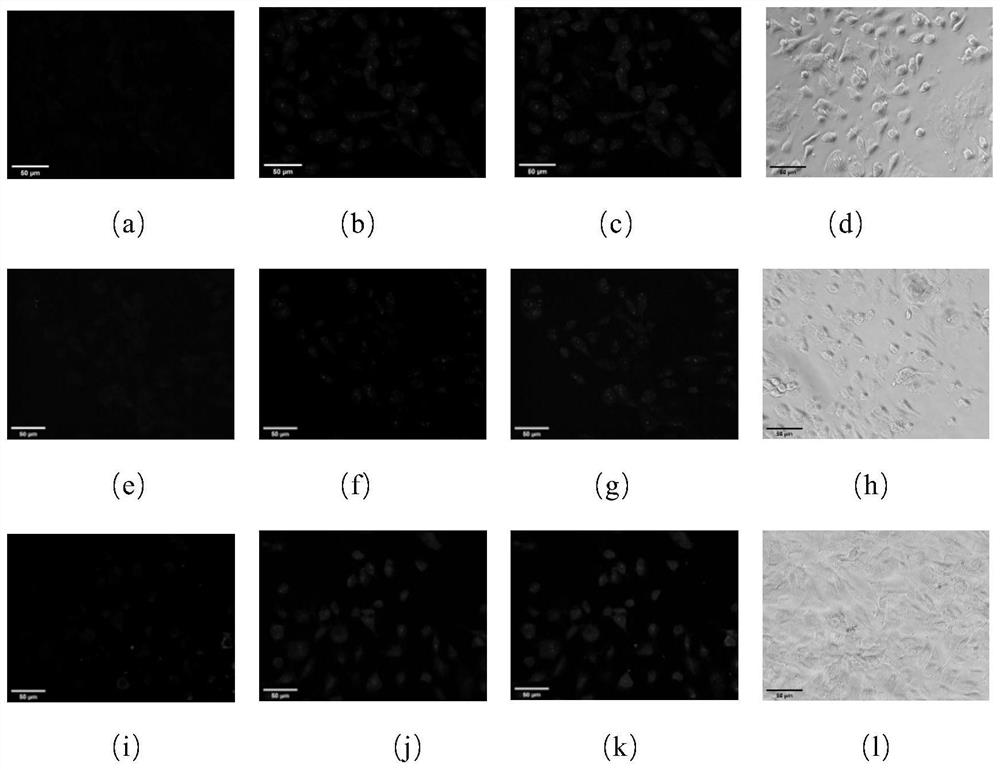
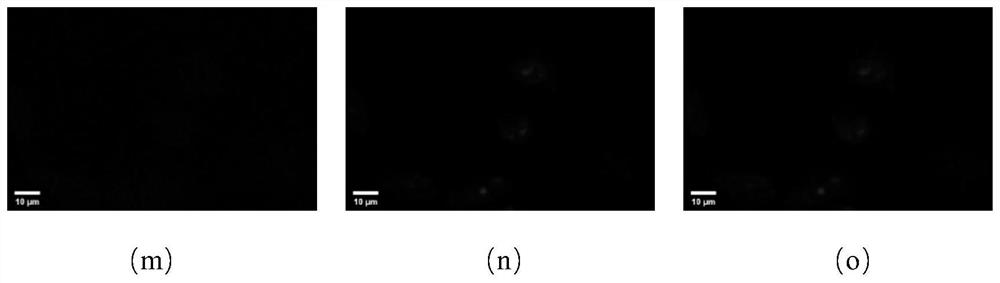
[0052] After the photoaffinity probe based on the triazole active molecule is incubated with the cells, the ultraviolet light makes the probe form an irreversible covalent bond with its target, and the fluorescent group (azidocoumarin) is connected to the fluorescent group (azidocoumarin) through click chemistry. Soybein emits blue fluorescence, and the fluorescence localization is the action site of the probe. According to the preliminary experiment, the experimental parameters were determined as follows: the concentration ratio of fluorescein to photoaffinity probe was 1:1, and the concentration ratio of competitor to photoaffinity probe was 5:1, and the obtained results were relatively clear. For target protein confirmation experiments, select 4 μM photoaffinity probe, 4 μM azidocoumarin, 20 μM competitor, UV irradiation for 15 min and other param...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com