Trimeric quaternary ammonium salt surfactant constructed by dynamic imine bonds, preparation method and application thereof
A compound, selected technology, applied in the fields of botanical equipment and methods, preparation of organic compounds, preparation of imino compounds, etc., can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The preparation method of the present invention will be further described in detail below in conjunction with specific examples. It should be understood that the following embodiments are only illustrative and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies implemented based on the foregoing contents of the present invention are covered by the scope of the present invention.
[0104] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples, unless otherwise specified, can be obtained from commercial sources.
preparation example 1
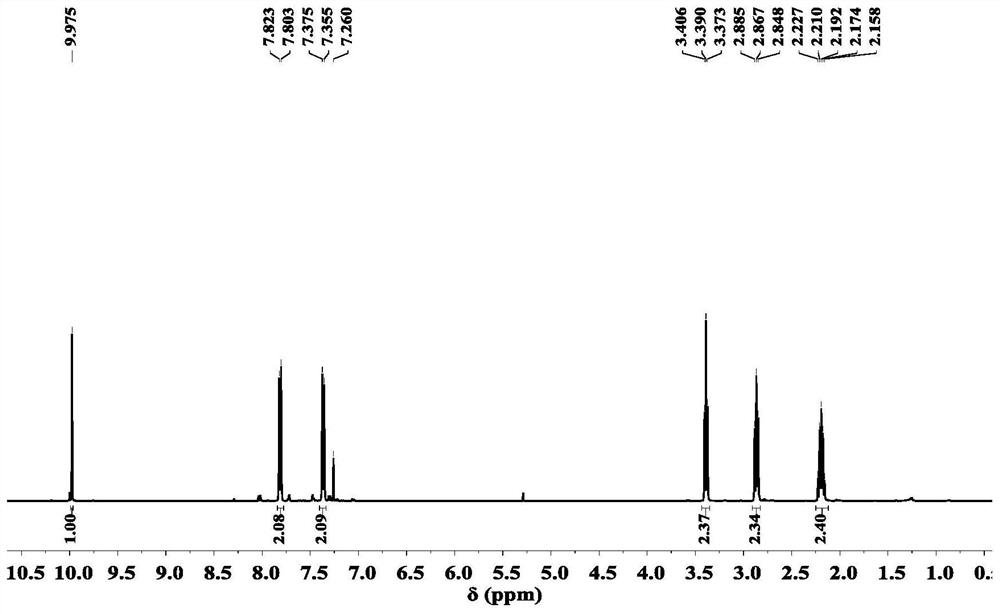
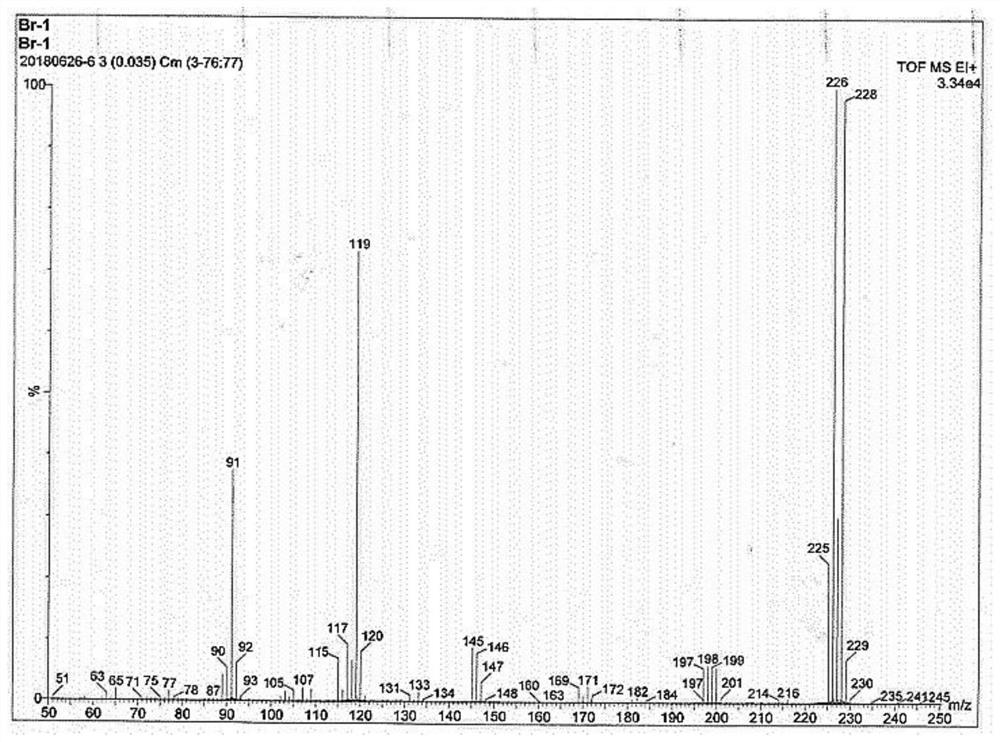
[0105] Preparation Example 1. Preparation of raw material 4-(3-bromopropyl)-benzaldehyde
[0106]
[0107] Dissolve 20g (100mmol) of 1-bromo-3-phenylpropane in 120mL of dichloromethane, cool to 0-5°C in an ice bath, and add 28.45g (150mmol) of TiCl 4 As a catalyst, keep the temperature at 0-2°C, and then slowly add the carbonylation reagent 1,1-dichloromethyl ether 10.44 (90mmol) to the mixed system dropwise. After the dropwise addition, continue to stir and react for 5 minutes, and slowly raise the temperature to 35°C Continue to stir for 15 min. After the reaction, the reaction mixture is slowly poured into a flask filled with ice water, and then transferred to a separatory funnel, extracted twice with dichloromethane to obtain the organic phase, and then saturated NaHCO 3 The organic phase was washed with aqueous solution, and the solvent was evaporated to obtain a crude product, which was then subjected to column chromatography (ethyl acetate / petroleum ether volume ratio 1:7 as...
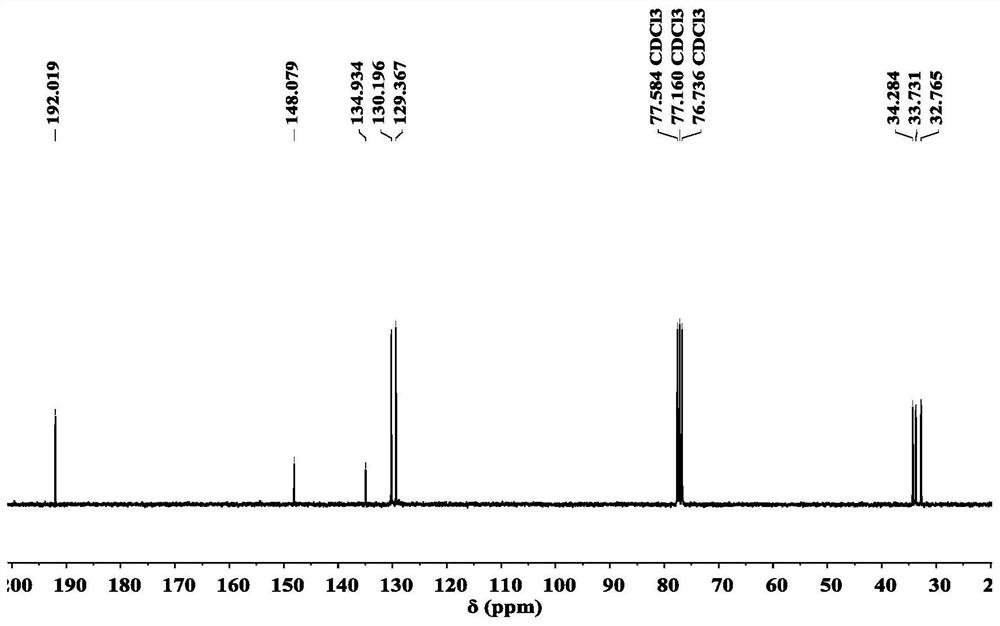
preparation example 2
[0112] Preparation Example 2. Quaternary ammonium salt compound TDA-(PhC=O) 3 Preparation
[0113]
[0114] Dissolve 2.76g (12mmol) of tris-(2-dimethylaminoethyl)amine in a 1:5 mixed solvent of 120ml ethanol and acetone, protect the reaction mixture with nitrogen, stir and heat to reflux temperature, then add 10.896g The raw material 4-(3-bromopropyl)-benzaldehyde (48 mmol) was added dropwise to the above mixture, and the reaction was continued for 48 hours after the addition was completed, until the reactants reached a higher conversion rate (the conversion rate of the reaction was monitored by mass spectrometry). After the reaction, the reaction mixture was cooled to room temperature, and acetone was added to wash the reaction mixture. After washing, the mixture is filtered to obtain a filter residue, and the filter residue is washed 3 times with the above-mentioned washing reagent. After filtration, the organic solvent is evaporated to dryness, and a yellow-brown solid crude p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com