Antibody targeting Sirp alpha or antigen binding fragment of antibody as well as preparation and application of antibody or antigen binding fragment
A technology for combining fragments and antibodies, applied in the field of biomedicine, which can solve the problems of low expression, the inability of antibodies to be combined at the same time, and the reduction of side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
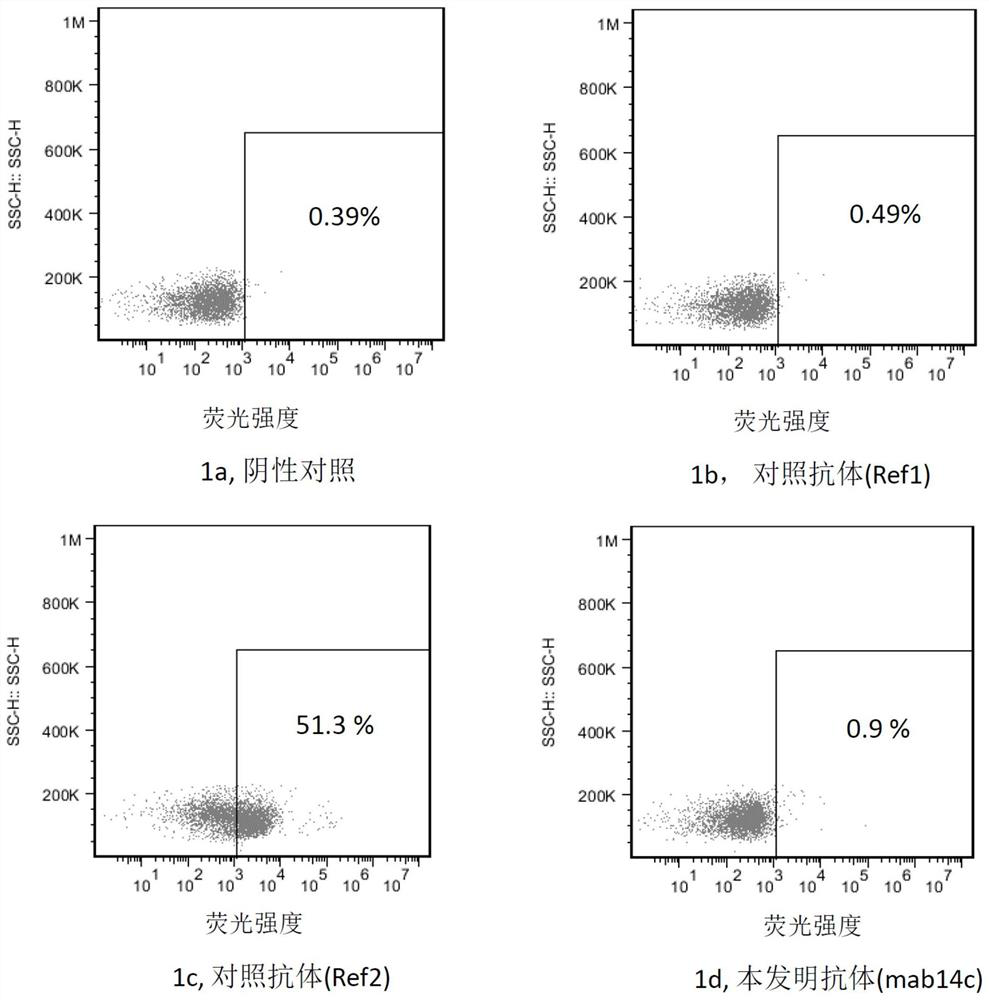
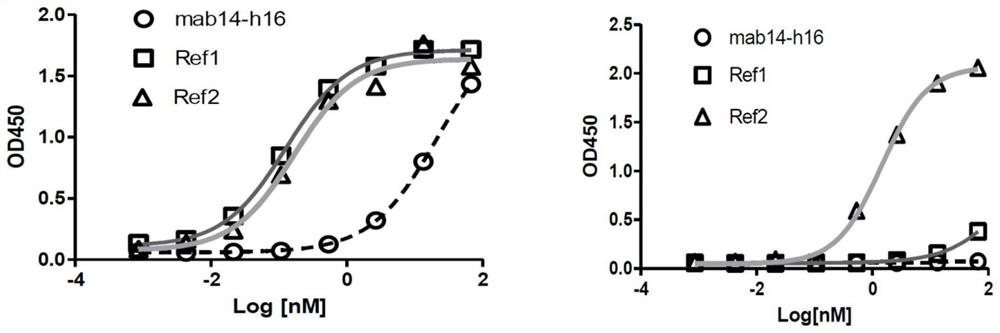
Image
Examples
Embodiment 1
[0130] Cloning, expression and purification of embodiment 1 antigen and antibody
[0131] Antigens used in the present invention may be purchased from different companies as follows.
[0132] Beijing Yiqiao Shenzhou Technology Co., Ltd.: Human Sirpα-V1-his (Cat. No.: 11612-H08H), Human Sirpα-V1-mFc (Cat. No.: 11612-H38H), Mouse Sirpα-his (Cat. No.: 50956-M08H), Human Sirpγ (Cat. No.: 11828-H08H); or Beijing Baipusaisi Biotechnology Co., Ltd.: human Sirpβ-hFc (Cat. No.: SIA-H5257), human Sirpγ-hFc (Cat. his (product number: B2048) or obtained by expression and purification of the present invention.
[0133] The expressed human Sirpα-V1 protein (his, or Fc Tag) sequence is NCBI Reference Sequence: NP_001035111, with a total length of 504 amino acids, of which the 1-30th is the signal peptide; the extracellular domain (ECD) is the 31-373rd amino acid. The 31st-137th amino acid of ECD is the Ig-like-V-type region, the 148th-247th amino acid is the Ig-like C1-type1 region, and t...
Embodiment 2
[0163] Embodiment 2 High expression cell line construction and cell viability (ELISA) detection
[0164] The high-expression cell lines used in the present invention are all constructed by the inventors through our company's stable cell line construction platform. The following takes the construction of human Sirpα high-expression cell lines as an example to illustrate the construction process. Specific steps are as follows:
[0165] On the first day of the experiment, 293T cells (Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences, Cat#: GNHu17) were seeded in two 6cm culture dishes, and the number of cells in each culture dish reached 7.5×10 5 . On the second day, add 4 μg of the encapsulating plasmid (pGag-pol, pVSV-G and other BioVector plasmid vector strains and cell gene collection centers) and the plasmid pBabe-hSirpα for cloning the human Sirpα gene into OPTI-MEM (Thermofisher Scientific, Cat#: 31985070) , to make the final volume 20...
Embodiment 3
[0169] Example 3 Anti-Sirpα antibody and antigen binding assay (ELISA)
[0170] The human Sirpα-V1-hFc described in Example 1, Sirpα-V1-his, Sirpα-V2-his, Sirpβ-his, Sirpγ-his, monkey Sirpα-his (cynoSirpα-his, His) or different antigens (recombinant proteins) such as NOD-mSirpα-his were diluted to 1 μg / ml, 2 μg / ml, or 5 μg / ml, and added to a 96-well microtiter plate (Corning, CLS3590-100EA) at a volume of 50 μl / well. placed in a 37°C incubator for 2 hours. After discarding the liquid, add 230 μl / well of 5% skimmed milk (bright skimmed milk powder) blocking solution diluted with PBS, incubate at 37°C for 3 hours or place at 4°C overnight (16-18 hours) for blocking. Discard the blocking solution and wash the plate 5 times with PBST buffer (PH7.4PBS containing 0.05% tweeen-20), then add 50μl / well supernatant (containing detection antibody) or 10μg / ml initial, 5-fold serial dilution of the antibody to be tested , incubate at 37°C for 1 hour, wash the plate 5 times with PBST, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com