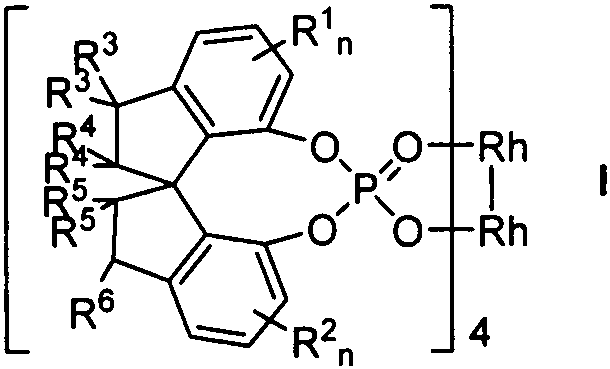
Chiral spiro phosphoric acid dirhodium complex as well as preparation method and application thereof
A technology of spirocyclic phosphoric acid and rhodium complexes, which is applied in the direction of rhodium organic compounds, organic compound/hydride/coordination complex catalysts, silicon organic compounds, etc., which can solve the problem of small differences in steric hindrance and difficulty in obtaining high enantioselectivity , Reaction type limitations and other issues, to achieve the effect of high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
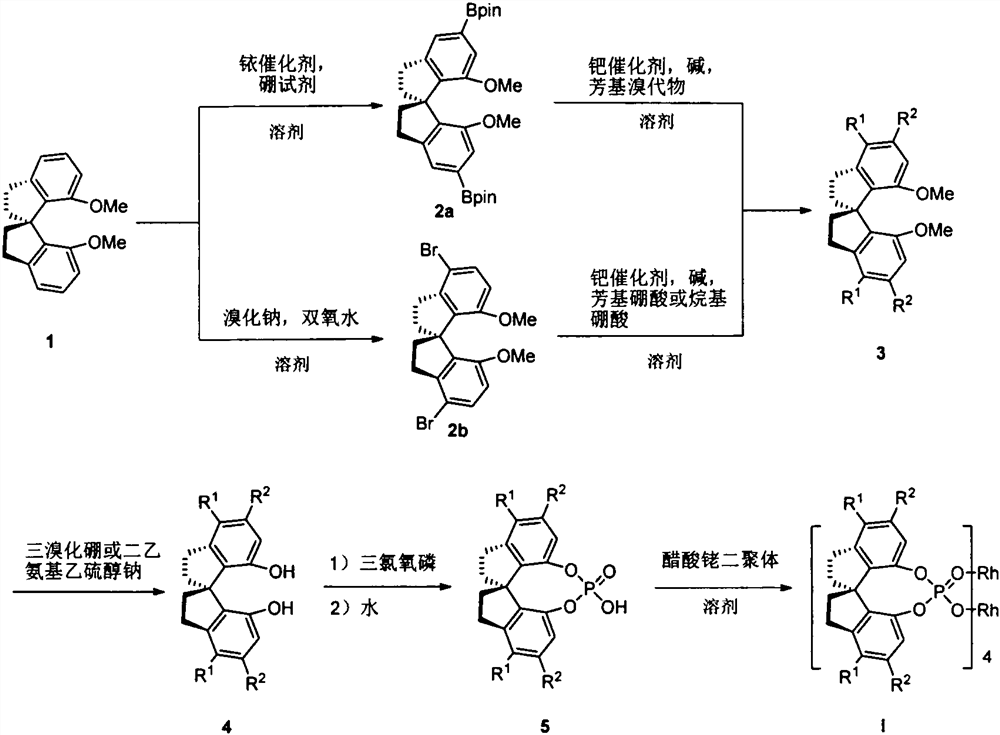
[0029] Synthesis of (S)-5,5'-bis(pinacol borate)-7,7'-bis(methoxy)-1,1'-spirobisdihydroindene (2a)
[0030]
[0031] Add compound 1 (2.3g, 8.0mmol), double pinacol borate (B 2 pin 2 , 4.5g, 17.6mmol), [Ir(OMe)(cod)] 2(212mg, 0.32mmol), and the ligand (151mg, 0.64mmol). The system was replaced by an argon atmosphere, dry tetrahydrofuran (30 mL) was added with a syringe, and stirred evenly. The oil bath was heated to 80° C. to react for 8 hours, and the reaction was complete as monitored by TLC. Cool to room temperature, remove the solvent with a rotary evaporator, and the residue is subjected to silica gel column chromatography (dichloromethane) to obtain 3.8 g of crude product, with a crude yield of 90%. Heating and recrystallization in redistilled petroleum ether gives pure White solid 2a, yield 70%, melting point: 190-192°C, [α] D 25 =-34.0 (c 1.0, CHCl 3 ).
[0032] 1 H NMR (400MHz, CDCl 3 )
[0033] δ7.34(s, 2H, ArH), 7.04(s, 2H, ArH), 3.56(s, 6H, 2OCH 3 ...
Embodiment 2
[0041] Synthesis of (S)-5,5'-diphenyl-7,7'-bis(methoxy)-1,1'-spirobisdihydroindene (3a)
[0042]
[0043] Add compound 2a (1.3g, 2.5mmol), bromobenzene (PhBr, 2.36g, 15.0mmol) and tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 , 443mg, 0.375mmol), after replacing argon, add toluene (30mL), ethanol (14mL) and potassium carbonate aqueous solution (1N, 20mL) successively, freeze and degas, and then place the reaction system in a 90°C oil bath to heat Stir overnight. After the completion of the reaction as monitored by TLC, cool to room temperature and dilute with ethyl acetate, separate the layers, extract the aqueous phase with ethyl acetate (3×20 mL), combine the organic phases and wash with saturated brine, and dry over anhydrous magnesium sulfate. After suction filtration and precipitation, silica gel column chromatography (petroleum ether / dichloromethane=4:1) gave 0.8 g of white solid 3a, yield 78%, melting point: 58-60 °C, [α] D 25 =-1.4 (c 1.0, CHCl 3 ).
[00...
Embodiment 3
[0051] Synthesis of (S)-5,5'-diphenyl-1,1'-spirobisdihydroindane-7,7'-diol (4a)
[0052]
[0053] Compound 3a (0.85 g, 2.0 mmol) was added into a 250 mL dry three-neck flask equipped with magnetic stirring, replaced with an argon atmosphere, dry dichloromethane (20 mL) was added with a syringe, and stirred evenly. The system was cooled to -78°C, and a solution of boron tribromide in dichloromethane (BBr 3 , 1M, 15mL), naturally return to room temperature, and stir overnight. The completion of the reaction was monitored by TLC, dichloromethane was added to dilute the reaction system, the organic phases were combined, washed successively with saturated sodium bisulfite solution, saturated sodium bicarbonate solution and saturated brine, and dried over anhydrous magnesium sulfate. Suction filtration, precipitation, and silica gel column chromatography (petroleum ether / dichloromethane = 1:1) yielded 0.46 g of white solid 4a, yield 57%, melting point: 101-102°C. [α] D 25 =+4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com