Glucose dehydrogenase mutant with chemical tolerance and application thereof in coenzyme regeneration
A technology of glucose dehydrogenase, mutants, applied in the directions of application, oxidoreductase, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
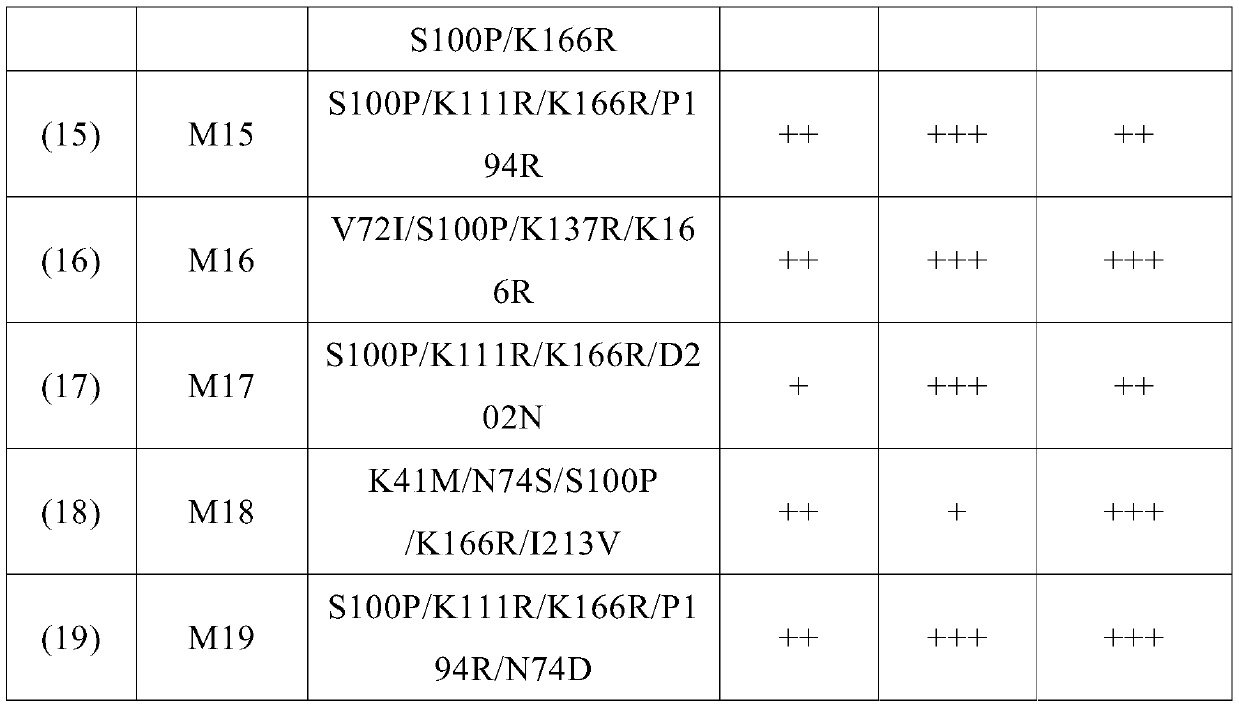
Image
Examples
Embodiment 1
[0058] Example 1 Random mutation screening for BmGDH mutants with increased tolerance to high concentrations of phenylethanol
[0059] Because the site that affects the stability of the enzyme is generally on the surface or loop structure of the enzyme, it is difficult to modify it in a rational or semi-rational way. The present invention uses a random mutation strategy to improve the tolerance of the enzyme to high concentrations of phenylethanol sex.
[0060] According to the open reading frame of BmGDH, the upstream and downstream primers were designed as follows:
[0061] Upstream primer, as shown in SEQ ID No.3:
[0062] GGGAATTC CATATG ATGTATAAAGATTTAG
[0063] Downstream primer, as shown in SEQ ID No.4:
[0064] CGC GGATCC TTATCCGCGTCCTGCTTG
[0065] The sequence shown underlined in the upstream primer is the restriction site of Nde I, and the sequence shown in the underline of the downstream primer is the restriction site of BamH I.
[0066] Using the plasmid p...
Embodiment 2
[0069] Example 2 DNA shuffling of beneficial mutants of BmGDH
[0070] On the basis of the mutations in Example 1, the dominant mutants obtained by error-prone PCR were randomly combined by DNA shuffling. The first step is to extract recombinant plasmids from these dominant mutants, and use equimolar mixtures as templates to amplify mutant genes by PCR. PCR reaction procedures: (1) Pre-denaturation at 95°C for 5 minutes; (2) Denaturation at 95°C for 1 minute; (3) Anneal at 55°C for 30 s; (4) Extend at 72°C for 1 min; Steps (2) to (4) are carried out for 30 cycles; finally extend at 72°C for 10 min, and store the product at 4°C. The PCR-amplified products of the mixed plasmids were identified by agarose gel electrophoresis, and the bands with the same size as the target fragment were purified and recovered with a gel extraction kit. The concentration of the target fragment after concentration and recovery should not be lower than 200 ng / μL. The second step is fragmentation of ...
Embodiment 3
[0095] Example 3 BmGDH expression and activity assay
[0096] Inoculate the recombinant Escherichia coli E. coli BL21(DE3) / pET28a-BmGDH into 4mL LB medium containing 50μg / mL kanamycin, and inoculate the bacterial liquid with 1% inoculum after shaking culture at 37°C overnight Incubate in a 250mL Erlenmeyer flask containing 50mL LB liquid medium (containing 50μg / mL kanamycin) at 37°C for 2-3h. Then inoculate 50mL of the bacterial liquid into a 2L Erlenmeyer shake flask containing 600mL of TB liquid medium, incubate at 37°C for 2-3h, then add 0.2mM IPTG inducer for induction, and incubate at 16°C for 20-24h. After induction, the cells were collected by a high-speed refrigerated centrifuge (4°C, 12000×g, 10 min). The collected wet cells were resuspended with excess physiological saline, centrifuged at 12000×g at 4°C for 10 min, and washed 2-3 times with the same operation. Weigh the collected wet bacteria and resuspend according to the ratio of adding 10mL of PBS (10mM, pH 6.5)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com