Preparation method of complex iron catalyst for wet desulphurization
A wet desulfurization and catalyst technology, which is applied in the preparation of organic compounds, catalyst protection, chemical instruments and methods, etc., can solve the problems of high desulfurization cost, increased suspended solids in the desulfurization system, blockage of fillers, and strong corrosiveness. Sulfur capacity, inhibit the production of secondary salts, good effect of absorbing hydrogen sulfide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
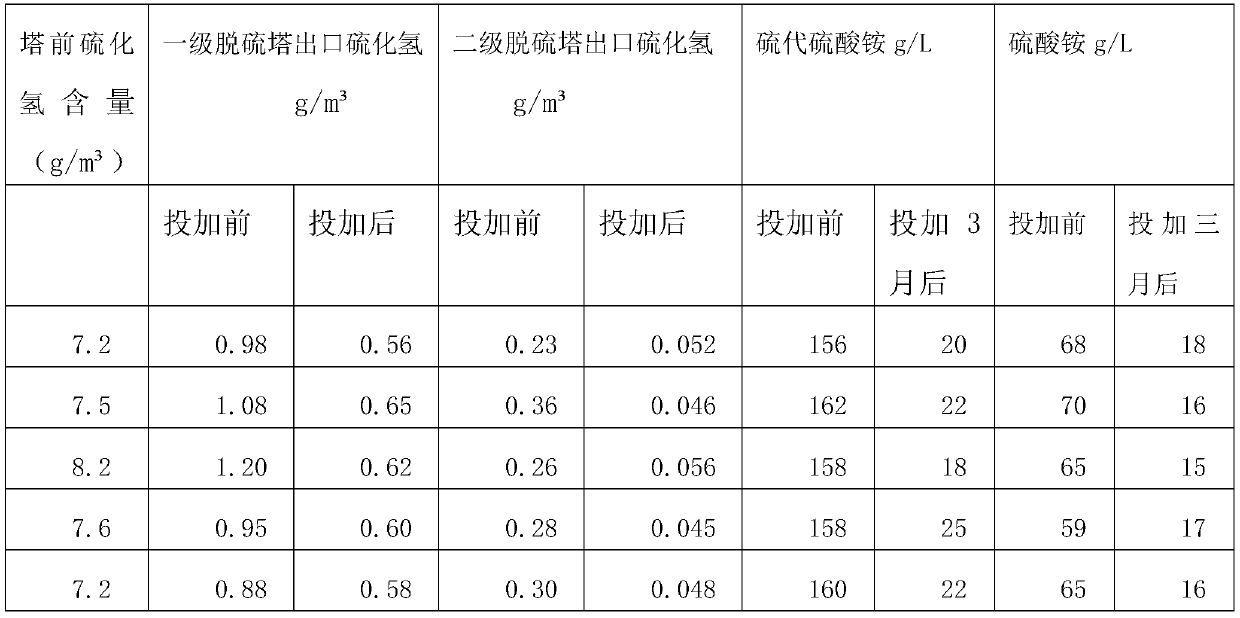
[0028] The preparation method that is used for wet method desulfurization complex iron catalyst comprises the following steps:
[0029] (1) Throwing phthalic anhydride and fuming sulfuric acid with a mass ratio of 1.5:1 into the reaction kettle and raising the temperature to 240°C until the reaction is complete to obtain sulfophthalic acid;
[0030] (2) Add sodium hydroxide to sulfophthalic acid, stir until the reaction is complete to obtain compound I;
[0031] (3) Compound I and cobalt chloride with a molar ratio of 1:2 were heated to 220°C to react to obtain Compound II;
[0032] (4) Compound I with a molar ratio of 1:2 and ferric hydroxide were raised to 220°C to react to obtain Compound III;
[0033] (5) heating compound II and compound III at a molar ratio of 1:1 to react at 260°C to obtain compound IV;
[0034] (6) Stabilizer preparation: mix ATMP, water, ammonia and citric acid with a mass ratio of 1:3:0.1:0.1 to make a double stabilizer;
[0035] (7) Compound IV wi...
Embodiment 2
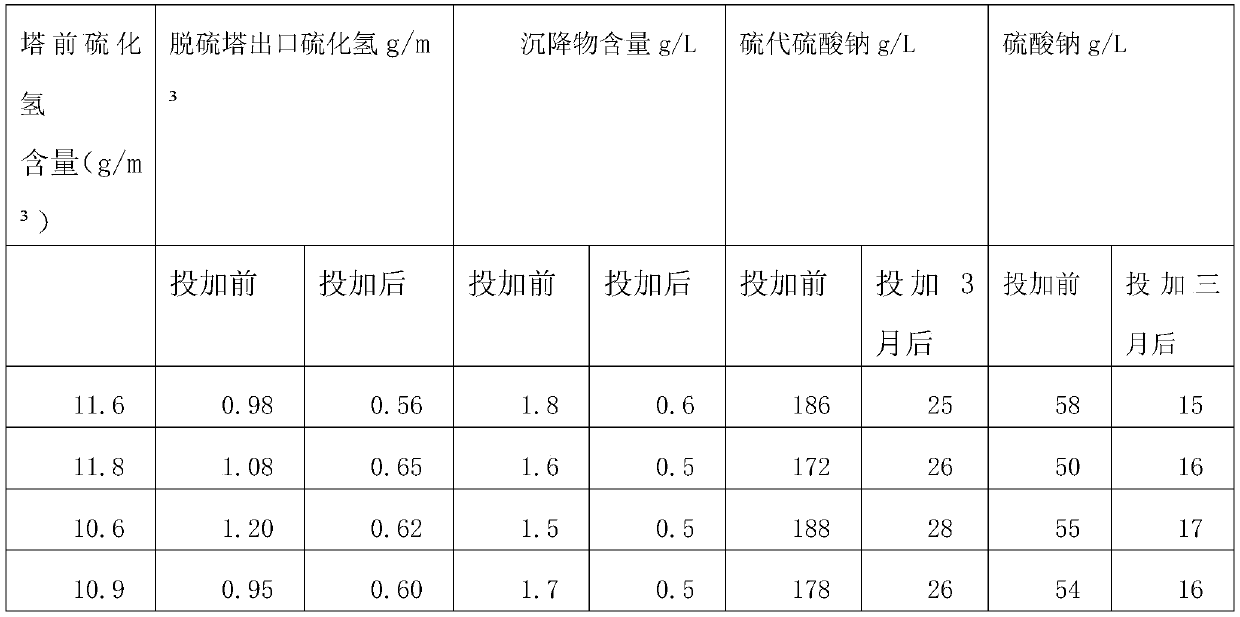
[0037] The preparation method that is used for wet method desulfurization complex iron catalyst comprises the following steps:
[0038] (1) Put phthalic anhydride and fuming sulfuric acid with a mass ratio of 1:1 into the reaction kettle and heat up to 220°C until the reaction is complete to obtain sulfophthalic acid;
[0039] (2) Add sodium hydroxide to sulfophthalic acid, stir until the reaction is complete to obtain compound I;
[0040] (3) Compound I with a molar ratio of 1:2 and cobalt chloride were heated to 200°C to react to obtain Compound II;
[0041] (4) The compound I with a molar ratio of 1:2 is reacted with ferric hydroxide to 240°C to obtain compound III;
[0042] (5) heating compound II and compound III at a molar ratio of 1:1 to 280° C. to react to obtain compound IV;
[0043] (6) Stabilizer preparation: mix ATMP, water, ammonia and citric acid with a mass ratio of 1:5:0.05:0.05 to make a double stabilizer;
[0044] (7) Compound IV with a mass ratio of 2:1 w...
Embodiment 3
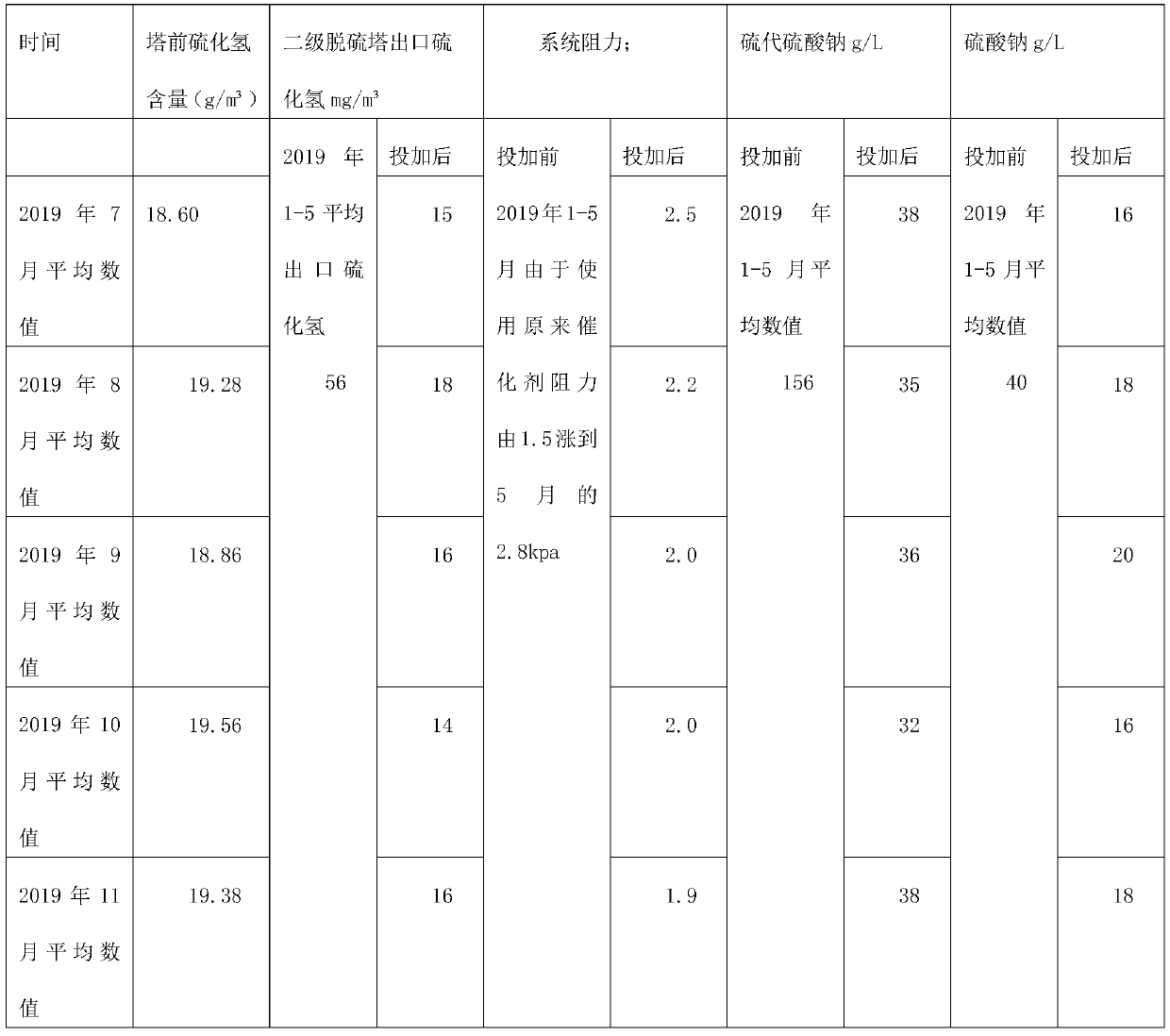
[0046] The preparation method that is used for wet method desulfurization complex iron catalyst comprises the following steps:
[0047] (1) Put phthalic anhydride and fuming sulfuric acid with a mass ratio of 2:1 into the reaction kettle and heat up to 240°C until the reaction is complete to obtain sulfophthalic acid;
[0048] (2) Add sodium hydroxide to sulfophthalic acid, stir until the reaction is complete to obtain compound I;
[0049] (3) Compound I with a molar ratio of 1:2 and cobalt chloride were heated to 240°C to react to obtain Compound II;
[0050] (4) react compound I with a molar ratio of 1:2 and ferric hydroxide to 200°C to obtain compound III;
[0051] (5) heating compound II and compound III at a molar ratio of 1:1 to react at 240°C to obtain compound IV;
[0052] (6) Stabilizer preparation: mix ATMP, water, ammonia and citric acid with a mass ratio of 1:1:0.15:0.1 to make a double stabilizer;
[0053] (7) Compound IV with a mass ratio of 5:1 is mixed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com