Monomer for biomass benzoxazine shape memory resin as well as preparation method and application of monomer
A benzoxazine and biomass technology, applied in the field of biomass benzoxazine shape memory resin monomer and its preparation, to achieve the effects of easy industrialized large-scale production, avoiding the use of solvents, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of aldehyde-containing biomass benzoxazine monomer
[0047] Mix 9.71g of furfurylamine and 6.00g of paraformaldehyde (CAS#: 30525-89-4), stir at room temperature for 15min, then add 15.22g of vanillin, stir at 85°C for 5h, and cool naturally to room temperature, adding ethanol for recrystallization to remove impurities, and drying the solid to obtain the aldehyde-containing biomass benzoxazine monomer.
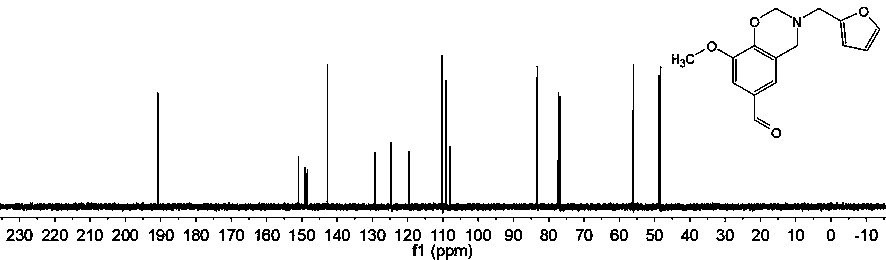
[0048] In this example, the synthetic reaction formula, hydrogen nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum of the aldehyde-containing biomass benzoxazine monomer are respectively referred to in the attached figure 1 , figure 2 and image 3 .
[0049] See attached figure 2 , it is the hydrogen nuclear magnetic resonance spectrum based on the aldehyde-containing biomass benzoxazine monomer provided by Example 1 of the present invention, about 9.81ppm represents the active H on the aldehyde group, and about 5.0...
Embodiment 2
[0074] (1) Preparation of aldehyde-containing biomass benzoxazine monomer
[0075] Mix 9.71g of furfurylamine and 6.60g of paraformaldehyde, stir at room temperature for 15min, then add 15.22g of vanillin, the reaction system is stirred and reacted at a temperature of 85°C for 5h, naturally cooled to room temperature, ethanol recrystallization to remove impurities , to obtain aldehyde-containing biomass benzoxazine monomer after drying.
[0076] (2) Preparation of Schiff base biomass benzoxazine monomer
[0077] Mix 30.04g of aldehyde-containing biomass benzoxazine monomer and 12.65g of polyetheramine D-230 (molecular weight: 230), react at a temperature of 125°C for 1.5h, cool naturally to room temperature, and dry to obtain Schiff base biomass benzoxazine monomer.
[0078] (3) Preparation of shape memory resin based on biomass benzoxazine
[0079] 11.0g Schiff base biomass benzoxazine monomer was put into the mold, the mold was put into an oven for degassing (10min at 150°C...
Embodiment 3
[0083] (1) Preparation of aldehyde-containing biomass benzoxazine monomer
[0084] Mix 9.71g of furfurylamine and 15g of 40wt% formaldehyde aqueous solution, stir at room temperature for 15min, then add 15.22g of vanillin, stir the reaction system at 85°C for 5h, cool naturally to room temperature, ethanol recrystallization to remove impurities , to obtain aldehyde-containing biomass benzoxazine monomer after drying.
[0085] (2) Preparation of Schiff base biomass benzoxazine monomer
[0086] Mix 27.31g of aldehyde-containing biomass benzoxazine monomer and 11.50g of polyetheramine D-230 (molecular weight: 230), react at a temperature of 125°C for 2h, cool naturally to room temperature, and dry to obtain the desired Fu base biomass benzoxazine monomer.
[0087] (3) Preparation of shape memory resin based on biomass benzoxazine
[0088] Put 10.5g Schiff base biomass benzoxazine monomer into the mold, put the mold into an oven for degassing (10min at 150°C), and then put the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Storage modulus | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com