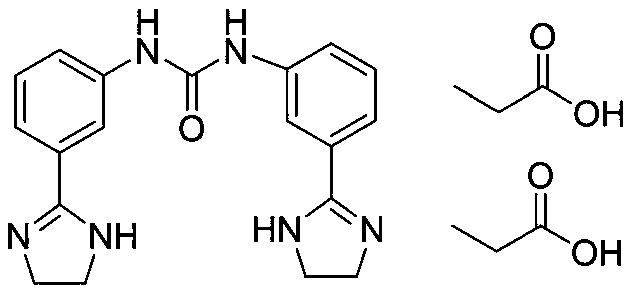
Preparation method of imidocarb dipropionate and intermediate thereof
A technology of imidazolium dipropionate and intermediates, which is applied in the field of preparation of imidazolium dipropionate, can solve the problems of not conforming to the concept of green synthesis, not suitable for industrial production, and high toxicity of diphosgene, and achieve the reduction of solid Effects of waste generation, ease of operation, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
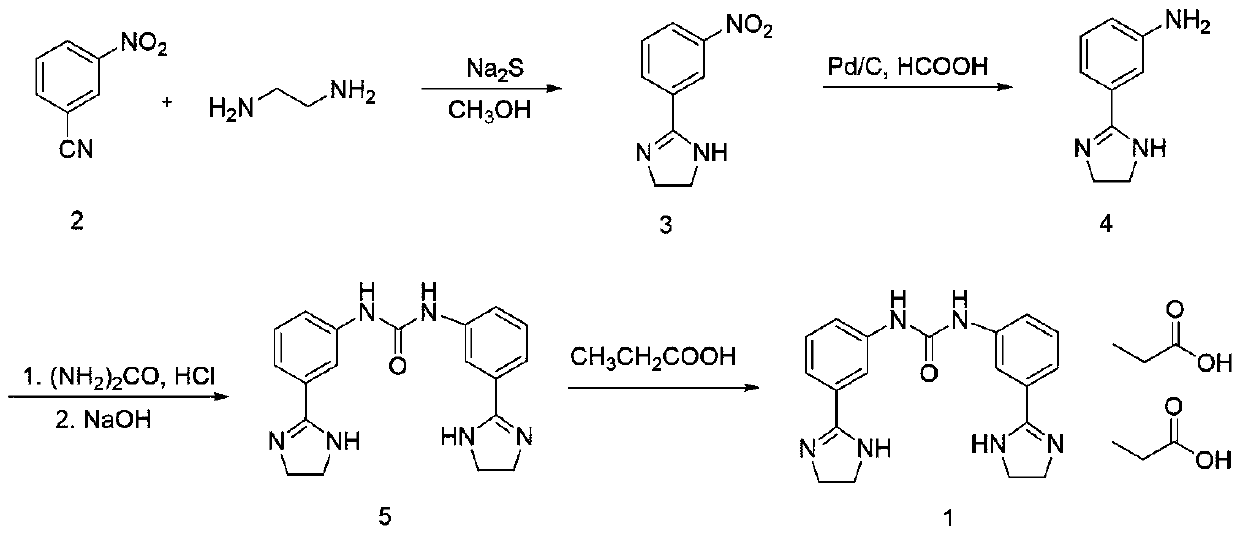
[0024] Synthesis of compound 3
[0025] Add 1L of methanol into a 2L three-necked reaction flask, add m-nitrobenzonitrile (100g, 0.67mol) under stirring, Na 2 S (26.35g, 0.335mol) and ethylenediamine (48.65g, 0.81mol) were heated and stirred to reflux. Reflux for 8 hours, heat filter, cool the filtrate to -5°C ~ 0°C, stir and crystallize for 2 hours, filter, wash the filter cake with a small amount of cold methanol, and dry the product in vacuum to obtain light yellow solid 2-(3-nitrophenyl) Imidazoline (123 g, 95.3%).
Embodiment 2
[0027] Synthesis of compound 3
[0028] Add 1L of methanol into a 2L three-necked reaction flask, add m-nitrobenzonitrile (100g, 0.67mol) under stirring, Na 2 S (52.7g, 0.67mol) and ethylenediamine (48.65g, 0.81mol) were heated and stirred to reflux. Reflux for 8 hours, heat filter, cool the filtrate to -5°C ~ 0°C, stir and crystallize for 2 hours, filter, wash the filter cake with a small amount of cold methanol, and dry the product in vacuum to obtain light yellow solid 2-(3-nitrophenyl) Imidazoline (120 g, 93.2%).
Embodiment 3
[0030] Synthesis of Compound 4
[0031] Add 1L of water, 2-(3-nitrophenyl)imidazoline (120g, 0.63mol) into a 3L reaction flask, carefully add 3.6g of 10% wet palladium carbon, slowly raise the temperature and stir to reflux, slowly add formic acid dropwise A solution of ammonium (119.7g, 1.89mol) in water (240mL) was added for about 0.5h, kept warm for 2h, filtered to obtain a light yellow clear liquid, and the filtrate was directly injected into the next step.
[0032] Synthesis of compound 5
[0033] Add the reaction solution from the previous step, hydrochloric acid (134mL, 1.6mol), and urea (19.2g, 0.32mol) into a 2L three-necked reaction flask in turn, heat and stir until reflux under nitrogen protection, keep the reaction for 8h, and cool the reaction solution to room temperature. / L of sodium hydroxide aqueous solution to adjust the pH to 9-10, a large amount of white solids precipitated, stirred at room temperature for 0.5h, filtered, washed the filter cake with a sma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com