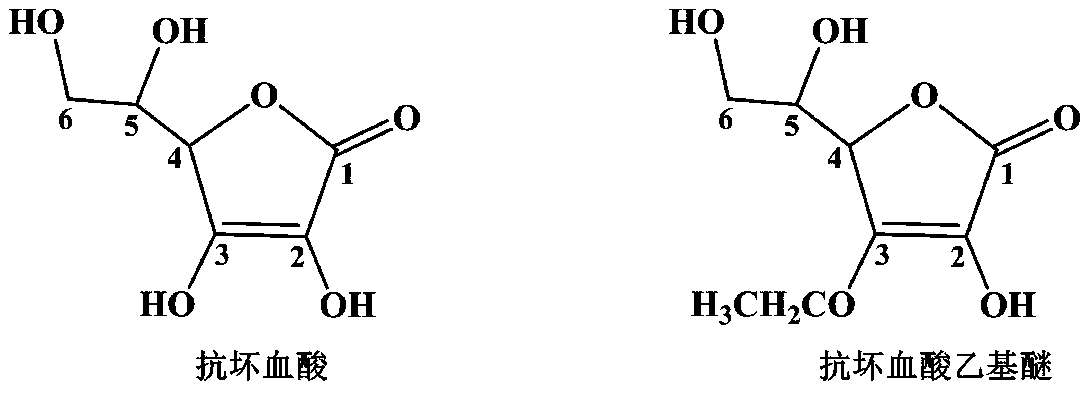
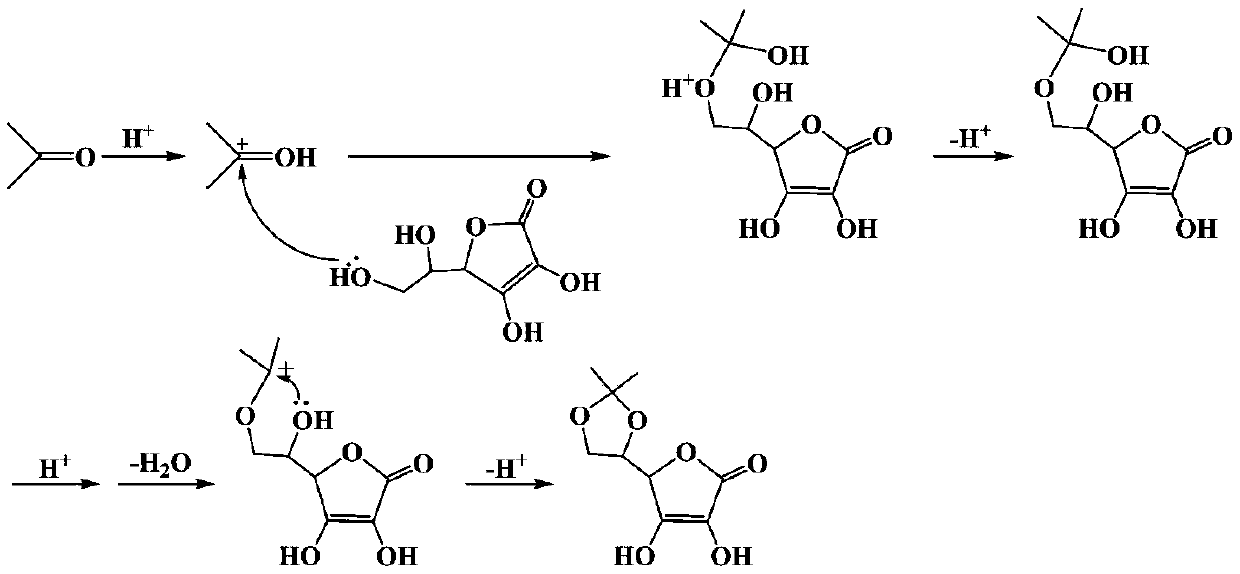
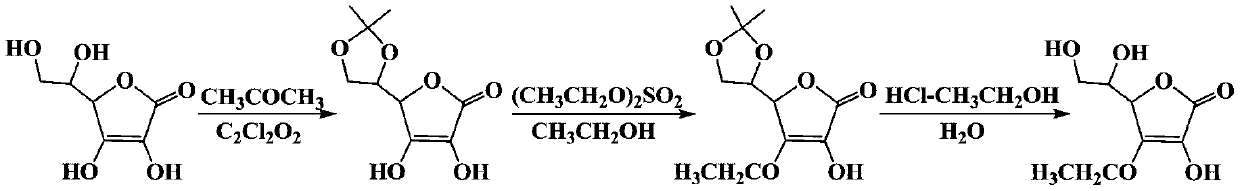Preparation process of ascorbyl ethyl ether
A technology for the preparation of ethyl ascorbic acid ether, which is applied in the production of organic chemistry and bulk chemicals, can solve the problems of increased production costs, many by-products, environmental pollution, etc., and achieve reduced production and treatment costs, reduced waste water discharge, and reduced The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of 5,6-O-isopropylidene-L-ascorbic acid
[0050] 176 g (1 mol) of ascorbic acid and 870 g (15 mol) of acetone were sequentially added into a 5L reactor, stirred for 10 minutes, 6.5 g (0.05 mol) of oxalyl chloride was added dropwise under ice-cooling conditions, and reacted at 30°C for 9 hours. After the reaction was completed, the filter cake and filtrate A were obtained after filtration. The filtrate A was used for later use. The filter cake was washed with 80 g of acetone and dried to obtain 195.1 g of 5,6-O-isopropylidene-L-ascorbic acid with a yield of 90.2%.
[0051] Add 74 grams of calcium oxide (1.32mol) to filtrate A, stir for 3 hours, filter, and use 90 grams of filtrate Molecular sieves were dried for 12 hours and filtered to obtain the treated acetone filtrate B, which can be reused.
[0052] 176 g of ascorbic acid was added to the treated acetone filtrate B, stirred for 10 minutes, 6.5 g of oxalyl chloride was added dropwise under ice...
Embodiment 2
[0055] Example 2 Preparation of 3-O-ethyl-5,6-O-isopropylidene-L-ascorbic acid
[0056] (1) Add 216.2 grams of 5,6-O-isopropylidene-L-ascorbic acid prepared in Example 1 into a 5L reactor, add 430 grams (9.3 mol) of ethanol, stir for 30 minutes, and add sodium bicarbonate 131 grams (1.56mol), slowly dropwise added 200 grams of diethyl sulfate (1.3mol), gradually heated to 55 ° C, reacted at this temperature for 6.5 hours, then concentrated the reaction solution, added 250 grams of dichloromethane (2.94mol ), filtered, and recrystallized to obtain 203.5 grams of 3-O-ethyl-5,6-O-isopropylidene-L-ascorbic acid, with a yield of 83.4%.
[0057] (2) Using the same method, use the ethylating reagent bromoethane instead of diethyl sulfate for operation, and explore the influence of different ethylating reagents on the yield.
[0058] Add 216.2 grams of 5,6-O-isopropylidene-L-ascorbic acid prepared in Example 1, 430 grams of ethanol, stir for 30 minutes, add 131 grams of sodium bicarb...
Embodiment 3
[0061] The preparation of embodiment 3 ascorbic acid ethyl ethers
[0062] Add 244 grams of 3-O-ethyl-5,6-O-isopropylidene-L-ascorbic acid, 320 grams of ethanol, stirred to dissolve, 55 grams of hydrogen chloride ethanol solution, water 30 grams, heated to 55 ° C, reacted for 4 hours, concentrated the reaction solution to one-third of the original volume, added an equal volume of dichloromethane for recrystallization, and obtained 190.4 grams of ascorbic acid ethyl ether, with a yield of 93.3%; Recrystallized with ethanol (volume ratio 2:1) to obtain 177.5 g of ascorbic acid ethyl ether (molecular weight: 204.18), with a yield of 86.9%.
[0063] Embodiment 3 gained ascorbic acid ethyl ether is carried out liquid chromatography and magnetic resonance analysis, and the results are as follows:
[0064] The purity determined by HPLC is 99.95%, 1 H-NMR (400MHz, CDCl 3 ):4.80(s,1H),4.42-4.44(m,2H),3.91-3.93(m,1H),3.61-3.63(m,2H),1.24-1.27(t,3H). 13 C-NMR (100MHz, CDCl 3 ): 173....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com