Application of mydgf protein in preparation of regulators for telomerase expression and cell senescence
A technology of cell aging and protein expression, applied in the field of telomerase, to achieve important application prospects, delay the decline of expression, and improve the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
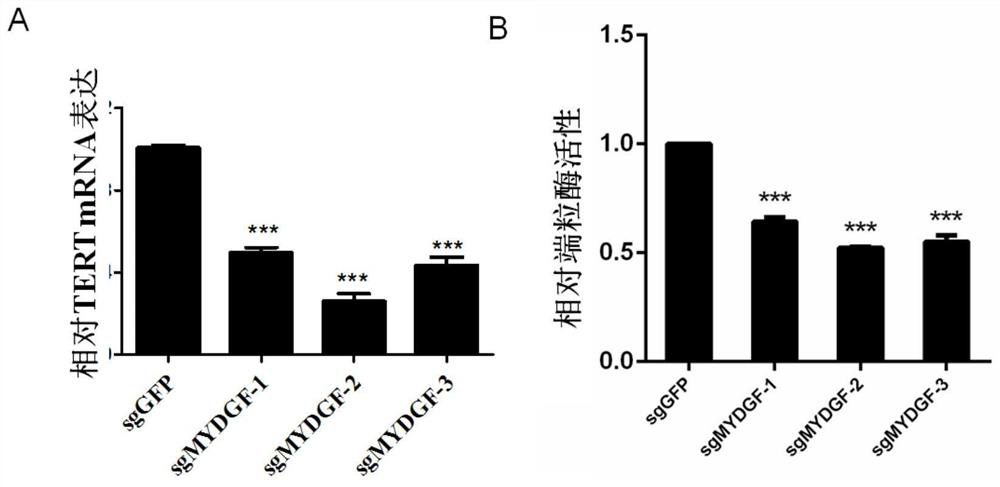
[0041] Example 1 Effect of MYDGF expression on telomerase activity in cells
[0042] 1. Method
[0043] 1. Knockout of MYDGF in wild-type Hela cells
[0044] Design and order gRNA template single-stranded complementary DNA. The PX330 vector was annealed and ligated, the strains were transformed and sequenced, the correct clone was selected, amplified and cultured to extract the plasmid, and the plasmid was transiently transferred to 293T cells for T7E1 digestion to identify the positive cut gRNA. The candidate gRNA complementary DNA was ligated to the lentiviral packaging vector pLenti-gRNA-puro with different resistances, and the 293T packaging lentivirus was used to infect HeLa cells stably expressing pLenti-inducible Cas9-neo. Stable cell lines were obtained by screening with 1 μg / mL puromycin puromycin, and 1 μg / mL doxycycline was added to induce and culture for 3-6 days. The cell growth was observed and the target protein was detected by western blotting to verify the k...
Embodiment 2
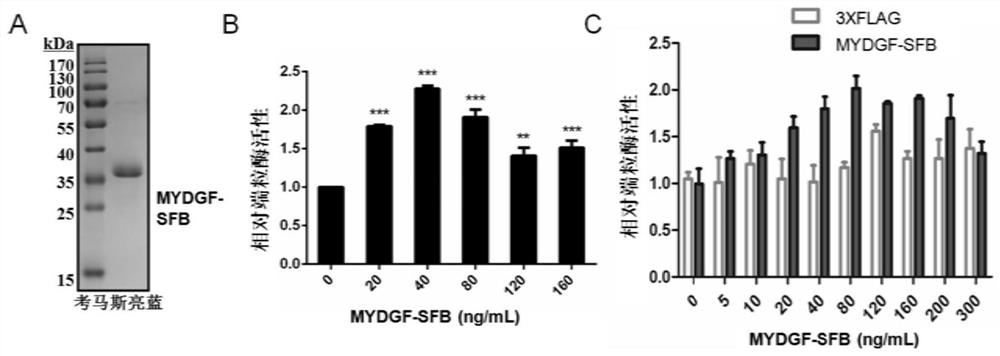
[0060] Example 2 MYDGF eukaryotic protein purification and its telomerase activity on HeLa and MSC
[0061] 1. MYDGF eukaryotic protein purification
[0062]The MYDGF-FLAG plasmid was transiently transfected into 293T cells and cultured in 15cm plates. Filter the medium with a 0.22um filter and separate the supernatant. The obtained cells were separated and purified using Anti-FLAG M2 affinity gel (company: Sigma). Take 200ul gel and take 1mL of 4 ℃ pre-cooled PBS solution to wash the column. Samples were loaded, and all supernatants passed through the cartridge at a natural flow rate, and the flow-through was collected for detection. Unbound protein was washed away by adding 1 mL of PBS solution. Add 300ul PBS solution containing 3×Flag (100ug / mL) to elute the target protein, wash 4 times, and collect the washing solution for protein detection. Add 600ul of 0.1M Glycine-HCl (pH3.5) to wash, and collect the filtrate for detection. Add 1 mL of PBS solution to wash off Gly...
Embodiment 3
[0067] Example 3 Purification of MYDGF prokaryotic protein and its telomerase activity on MSCs
[0068] 1. MYDGF prokaryotic protein purification
[0069] The obtained prokaryotic expression vectors (PET28-N-His-MYDGF, PET21b-MYDGF-C-His) were transformed into BL21 (DE3) competent cells, wherein PET28-MYDGF-N-His was Kana resistant, PET21b-MYDGF-C -His is Amp resistance. Pick a single clone into the corresponding 8 mL of resistant LB medium, and cultivate and activate it for 12 h at 37 °C and 120 rpm. Take 6 mL of activated bacterial liquid, add it to 300 mL of LB medium with corresponding resistance, and cultivate at 37 °C and 120 rpm for 4-6 h. During this period, take the bacterial liquid for OD600 absorbance detection. When the OD600 reaches 0.4-0.6, the strain enters logarithmic growth. period, at which point IPTG was added to a final concentration of 0.1 mM for induction. Afterwards, the bacterial liquid was transferred to a 16°C shaker at 120 rpm and cultured overnig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com