Method for detecting potential mutagenic impurities in pitavastatin calcium tablets
A technique for detecting pitavastatin calcium and its detection method, which is applied in the field of drug quality detection, can solve the problems of being unable to detect the content of impurity 1 and impurity 2, interfering with the detection of impurity 1 and impurity 2, etc., and achieve improved drug safety, good specificity, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of mobile phase A is as follows: weigh 0.6 g of acetic acid, dilute to 1 L with deionized water, and adjust the pH value to 3.5 with sodium acetate solution.
[0044] It has been proved by experiments that the organic filter membrane has an adsorption effect on the impurity 2, which will lead to a decrease in the chromatographic peak area. Therefore, centrifugation or standing should be used for sample treatment, and filter membrane filtration should not be used.
[0045] Preparation of impurity reference substance solution: Weigh 5.0mg each of impurity 1 and impurity 2, put in a 100mL measuring bottle, add solvent to dissolve and dilute to the mark, shake well, and use it as impurity reference substance mother liquor; take impurity reference substance mother liquor 3mL, put in 100mL volume bottle, dilute to the mark with a solvent, shake well, and use it as the stock solution of the impurity reference substance; take 2 mL of the stock solution of ...
Embodiment 1
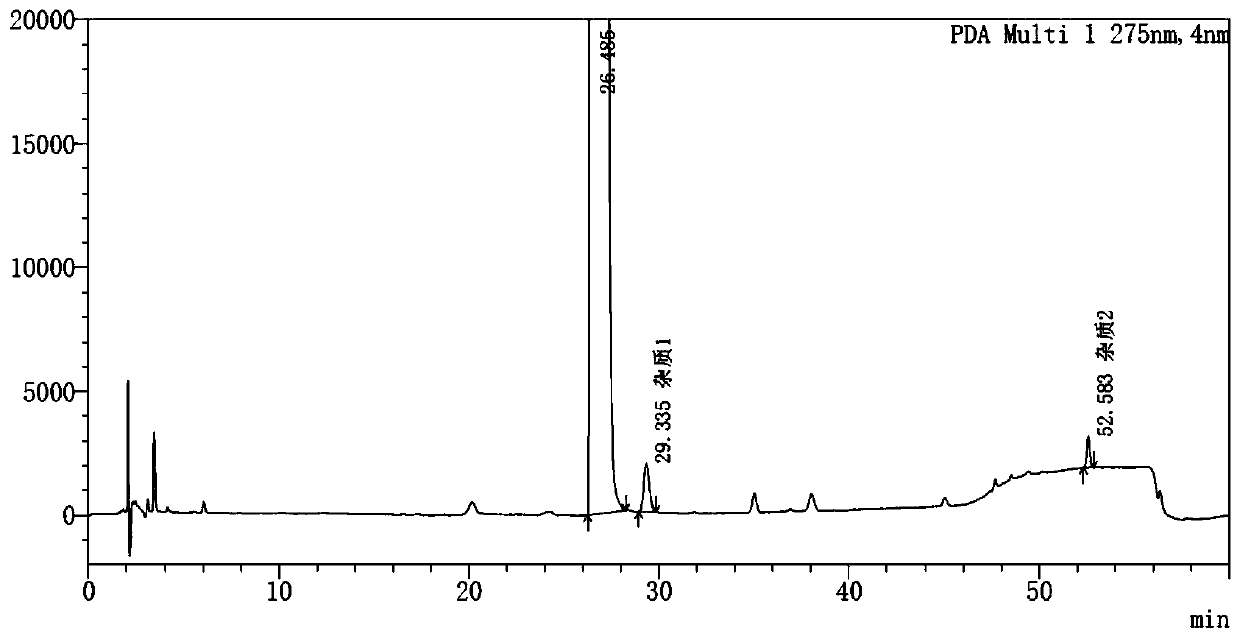
[0050] Good specificity:
[0051] It has been verified that (1) the solvent and blank excipients do not interfere with the detection of the main peak of pitavastatin calcium, impurity 1 peak, and impurity 2 peak; (2) the separation between the main peak and adjacent impurities and between adjacent impurities is not less than 1.5; (3) system suitability The retention time and resolution of each peak in the solution are shown in Table 2.
[0052] Table 2 System Suitability Solution Test Results
[0053]
Embodiment 2
[0055] Good system suitability:
[0056] The solvent does not interfere with the detection of each peak, the number of theoretical plates for the main peak is 15977; the number of theoretical plates for impurity 1 is 45800, and the tailing factor is 1.19; The RSDs of the peak areas of impurity 2 were all less than 1%. The results showed good systematicness.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com