Method for determining organic impurities in hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and organic impurities, applied in the field of drug analysis, can solve the problems of low sensitivity, missed detection of impurities, weak specificity of TLC method, etc., and achieve the effect of quality control and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
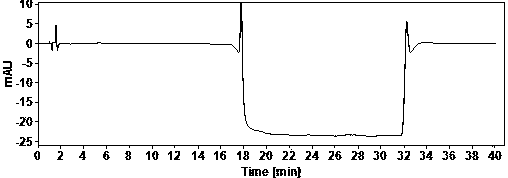
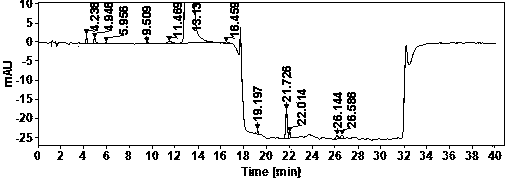
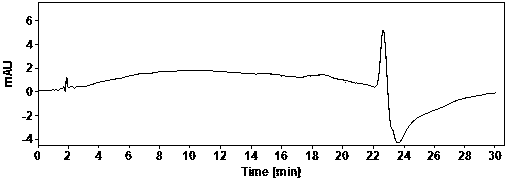
[0022] Example Determination of actual samples
[0023] (1) Use of instruments and reagents
[0024] Agilent technologies 1100 Series quaternary low-pressure liquid chromatograph (VWD detector), SartoriusBT25S and Sartorius BSA124S electronic balances, Shanghai Leici PHSJ-3F pH meter.
[0025] Glacial acetic acid (≥99.5%) and ammonia water (25%) are AR grade, acetonitrile is HPLC grade, water is China Resources C’estbon purified water, hydroxychloroquine sulfate (batch number: HCQ-20200428-01) is produced by Changsha Ruhong Pharmaceutical Technology Co., Ltd. Inc. provided.
[0026] (2) Chromatographic determination conditions
[0027] Chromatographic column: ACE Excel 5 Super C18, 4.6×150mm;
[0028] Column temperature: 30°C; Injection volume: 10ul; Flow rate: 1.0ml / min; Detection wavelength: 220nm;
[0029] Mobile phase A: NH at pH 10.0 3 -NH 4 + ; Mobile phase B: ammonia-acetonitrile solution.
[0030] The mobile phase was gradient eluted from mobile phase A and mob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com