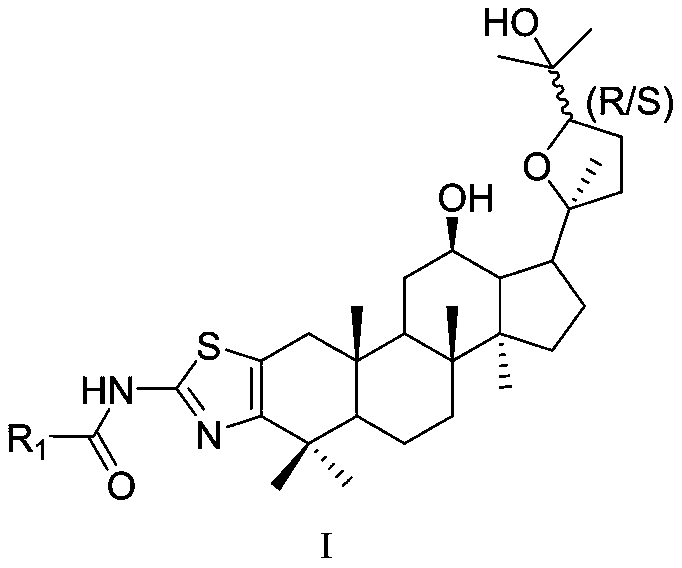
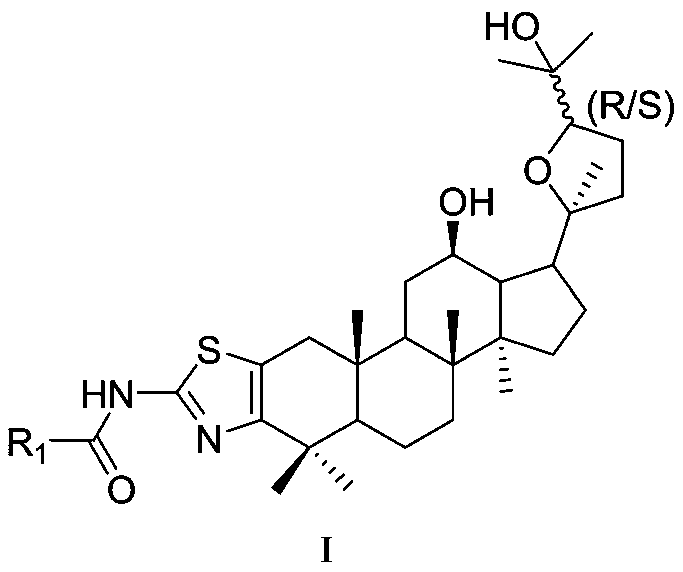
Ocotillol type ginseng sapogenin A ring-aminothiazole ring derivative and preparation method thereof
A technology of aminothiazole and ginseng soap, applied in the direction of drug combination, pharmaceutical formula, steroidal compounds, etc., can solve the problem that ginsenogenin has no anti-tumor activity, and achieve the effect of excellent anti-tumor activity and sensitivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
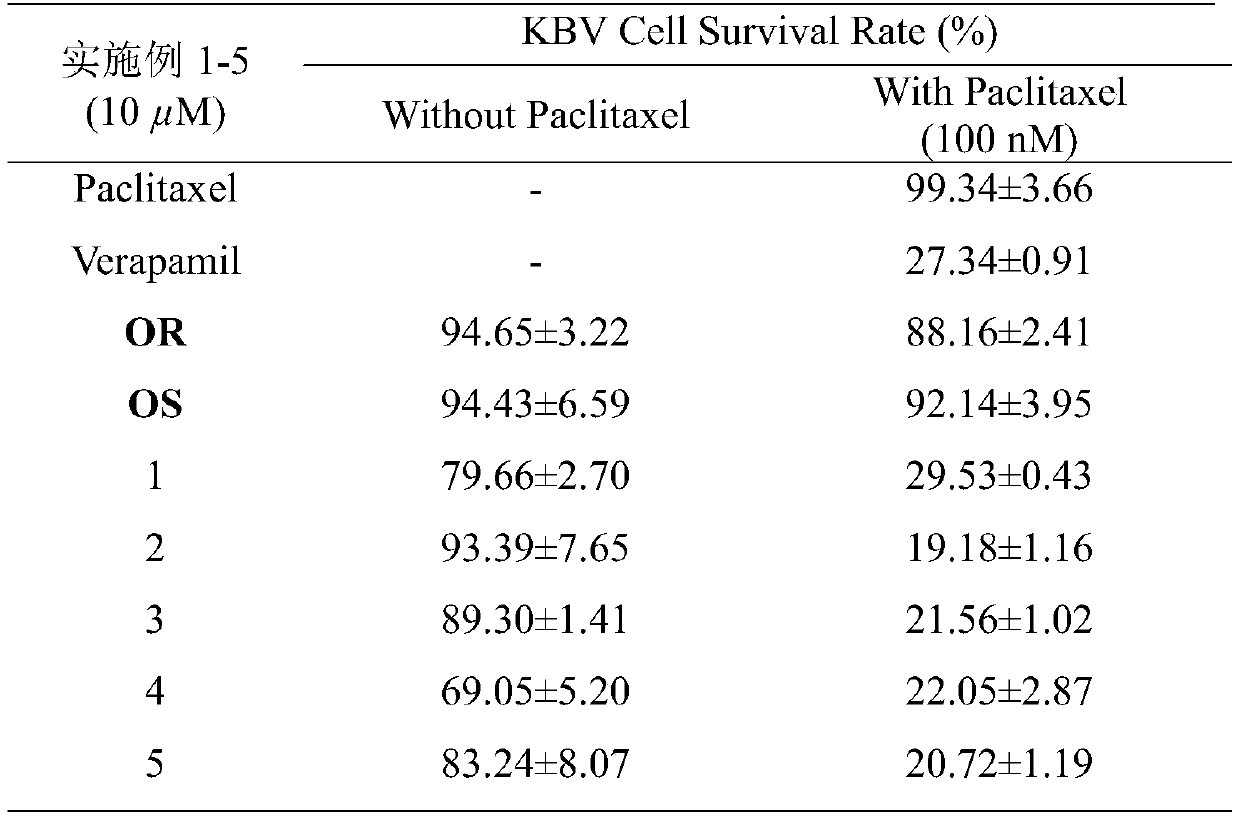
Examples
Embodiment 1
[0047] (20S,24R)-epoxy-12β,25-dihydroxy-2,3-[2,3-b]aminothiazole-N-alanyl-dammarane
[0048] Dissolve 20(S)-protopanaxadiol (5.0g, 10.9mmol) in chloroform (30.0mL), add DMAP (0.2g, 1.6mmol) and stir, then slowly add acetic anhydride (4.2mL, 44.3mmol) dropwise , stirred at room temperature for 1h. After rotary evaporation, dilute with ethyl acetate (100.0mL), wash with 10% hydrochloric acid until acidic, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, column chromatography (petroleum ether:ethyl acetate=10:1 ) to obtain 20(S)-3,12-diacetyl protopanaxadiol (5.1 g, 9.3 mmol, 85%) as a white solid.
[0049]Dissolve 20(S)-3,12-diacetyl protopanaxadiol (2.1g, 3.8mmol) in anhydrous dichloromethane (15.0mL), slowly add m-chloroperoxy A solution of benzoic acid (1.9 g, 75%, 10.7 mmol) in dichloromethane (15.0 mL) was raised to room temperature after 0.5 h and stirred for 2 h. Add isopropanol (1.0mL), continue to stir for 1 hour, ad...
Embodiment 2
[0056] (20S,24R)-epoxy-12β,25-dihydroxy-2,3-[2,3-b]aminothiazole-N-phenylalanyl-dammarane
[0057] The synthetic method was referred to Example 1 to obtain compound 2 (65.1 mg, 0.1 mmol, 85%) {(20S, 24R)-epoxy-12β, 25-dihydroxy-2,3-[2,3-b]amino Thiazole-N-benzoyl-dammarane} 1 H-NMR (CDCl 3 ,400MHz)δ(ppm):7.34–7.19(m,5H,Ar-H×5),3.89–3.81(m,1H,-OCH-),3.79(dd,J=9.4,3.9Hz,1H,NH 2 CH-), 3.55(td, J=10.4, 4.3Hz, 1H, -OCH-), 3.34(dd, J=13.9, 3.9Hz, 1H, Bn-H), 2.82–2.77(m, 1H, Bn- H), 2.73(d, J=13.0Hz, 1H, -CH 2 -),2.27(d,J=15.4Hz,1H,-CH 2 -),1.28(s,3H,-CH 3 ),1.26(s,3H,-CH 3 ),1.21(s,3H,-CH 3 ),1.13(s,3H,-CH 3 ),1.09(s,3H,-CH 3 ),1.03(s,3H,-CH 3 ),0.92(s,3H,-CH 3 ),0.89(s,3H,-CH 3 ).
Embodiment 3
[0059] (20S,24R)-epoxy-12β,25-dihydroxy-2,3-[2,3-b]aminothiazole-N-prolyl-dammarane
[0060] The synthetic method was referred to Example 1 to obtain compound 3 (64.0mg, 0.1mmol, 90%) {(20S,24R)-epoxy-12β,25-dihydroxy-2,3-[2,3-b]amino Thiazole-N-prolyl-dammarane} 1 H-NMR (CDCl 3 ,400MHz)δ(ppm):3.95–3.90(m,1H,-NHCHCO-),3.83(dd,J=8.8,6.8Hz,1H,-OCH-),3.54(td,J=10.5,4.5Hz, 1H, -OCH-), 3.06 (dt, J=10.2, 6.8Hz, 1H, -NHCH 2 -),2.97(dt,J=10.2,6.2Hz,1H,-NHCH 2 -),2.71(d,J=15.6Hz,1H,-CH 2 -),2.25(d,J=15.7Hz,1H,-CH 2 -),1.26(s,3H,-CH 3 ),1.25(s,3H,-CH 3 ),1.20(s,3H,-CH 3 ),1.13(s,3H,-CH 3 ),1.08(s,3H,-CH 3 ),1.02(s,3H,-CH 3 ),0.90(s,3H,-CH 3 ),0.87(s,3H,-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com