Brexpiprazole hydrochloride new crystal form and preparation method thereof
A technology of ebiprazole hydrochloride and ebiprazole hydrochloride, which is applied in the field of new crystal forms of ebiprazole hydrochloride and its preparation, can solve problems such as time-consuming filtration steps, affecting the health of workers, and affecting production progress, so as to avoid Potential safety hazards and health risks, beneficial to large-scale production, and the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0036] Preparation Example 1: Preparation of Ebiprazole Crude Product
[0037] Adopt the method described in CN101155804A Example 1 to prepare the crude product of ebiprazole: 90g 7-(4-chlorobutoxy)-1H-quinolin-2-one, 100g 1-benzo[b]thiophen-4-yl- A mixture of piperazine hydrochloride, 140g potassium carbonate, 60g potassium iodide and 900mL DMF was stirred at 80°C for 2h, water was added to the reaction solution, and crystals were precipitated, separated by filtration and dried to obtain 130g of crude ebiprazole with a purity of 95.6%.
Embodiment 1
[0038] Embodiment 1: the impact of glacial acetic acid, ethanol addition on the dissolution of ebiprazole
[0039] When other conditions are the same, observe the influence of the adding amount of glacial acetic acid and 95% ethanol on the dissolution of ebiprazole.
[0040] Add 100 g of ebiprazole crude product prepared in Preparation Example 1 to the reaction flask, add 95% ethanol and glacial acetic acid as shown in Table 1 below, stir at room temperature, and observe the dissolution of ebiprazole.
[0041] When ebiprazole is completely dissolved, heat up to 45°C, add 1N HCl 310mL, stir for 30-60 minutes after the addition, cool down to 15°C, continue stirring and crystallizing for 30-60 minutes, filter the solid, wash with ethanol, and dry to obtain hydrochloric acid Ebiprazole, the results are shown in Table 1.
[0042] Table 1 The impact of glacial acetic acid and ethanol addition on the dissolution of ebiprazole
[0043]
[0044] The results show that when the amou...
Embodiment 2
[0047] Embodiment 2: the influence of crystallization temperature on the yield of ebiprazole hydrochloride
[0048]Add 100 g of crude ebiprazole (prepared in Preparation Example 1), 4 L of 95% ethanol and 300 mL of glacial acetic acid to the reaction flask, stir at room temperature to dissolve the ebiprazole, heat up to the crystallization temperature, add 230 mL of 1N HCl, and finish adding Afterwards, solids were precipitated, and the stirring was continued for 30-60 minutes, the temperature was lowered to 15° C., the stirring was continued, filtered, washed with ethanol, and dried to obtain ebiprazole hydrochloride.
[0049] The impact of table 2 crystallization temperature on ebiprazole hydrochloride crystal particle size and yield, purity
[0050]
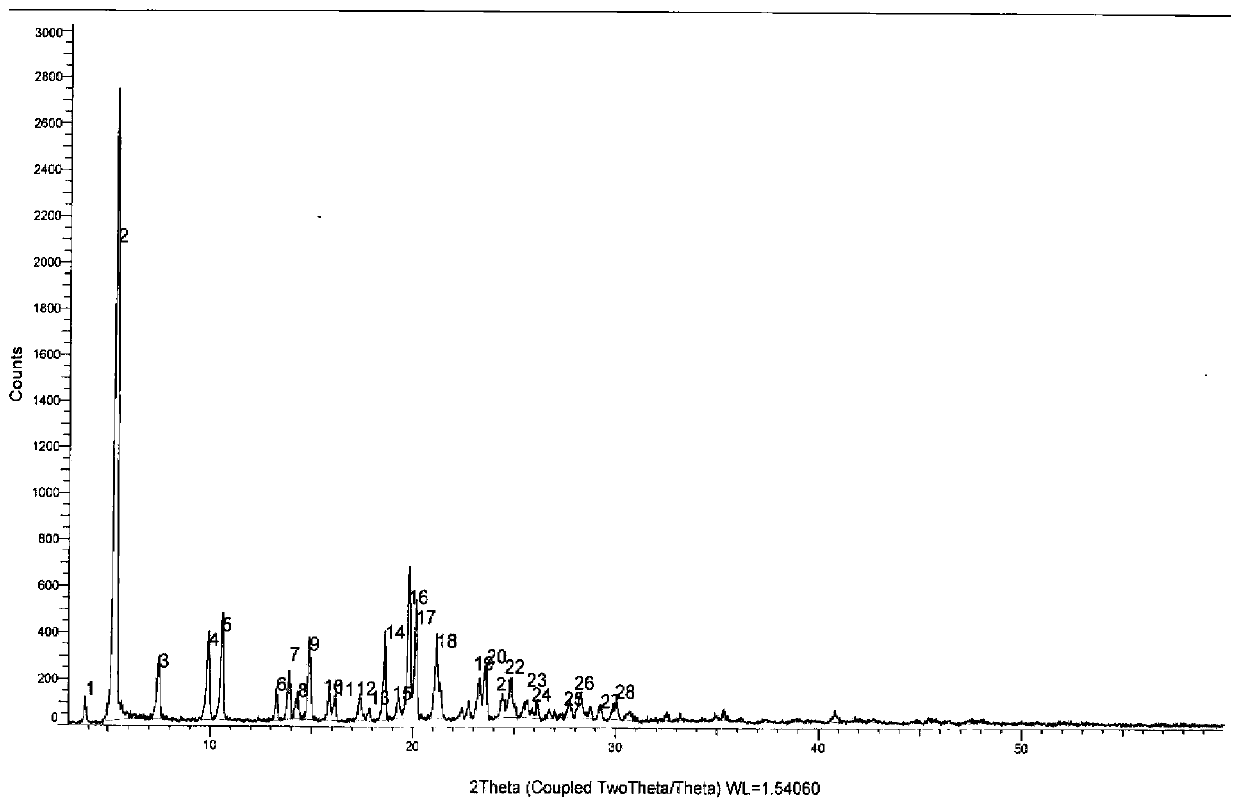
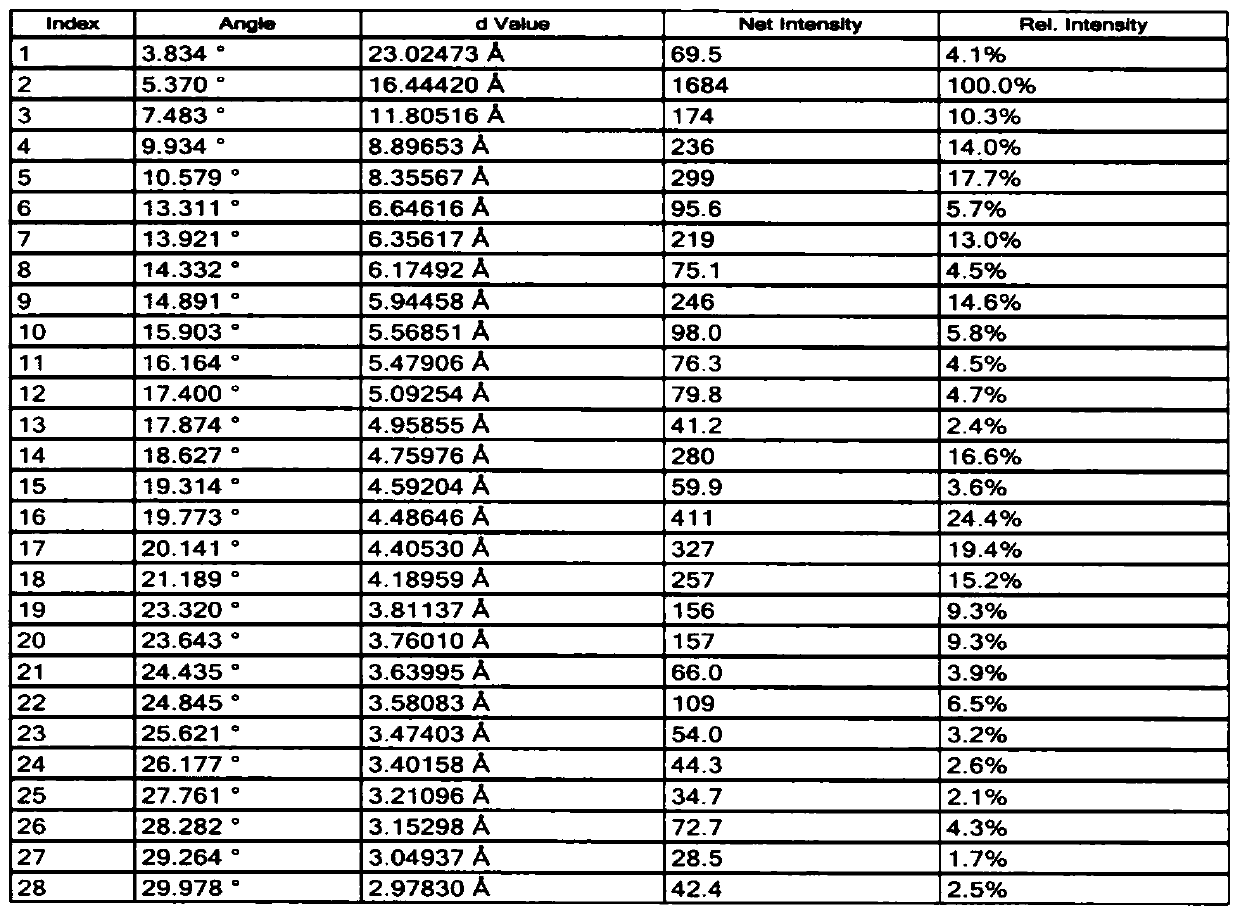
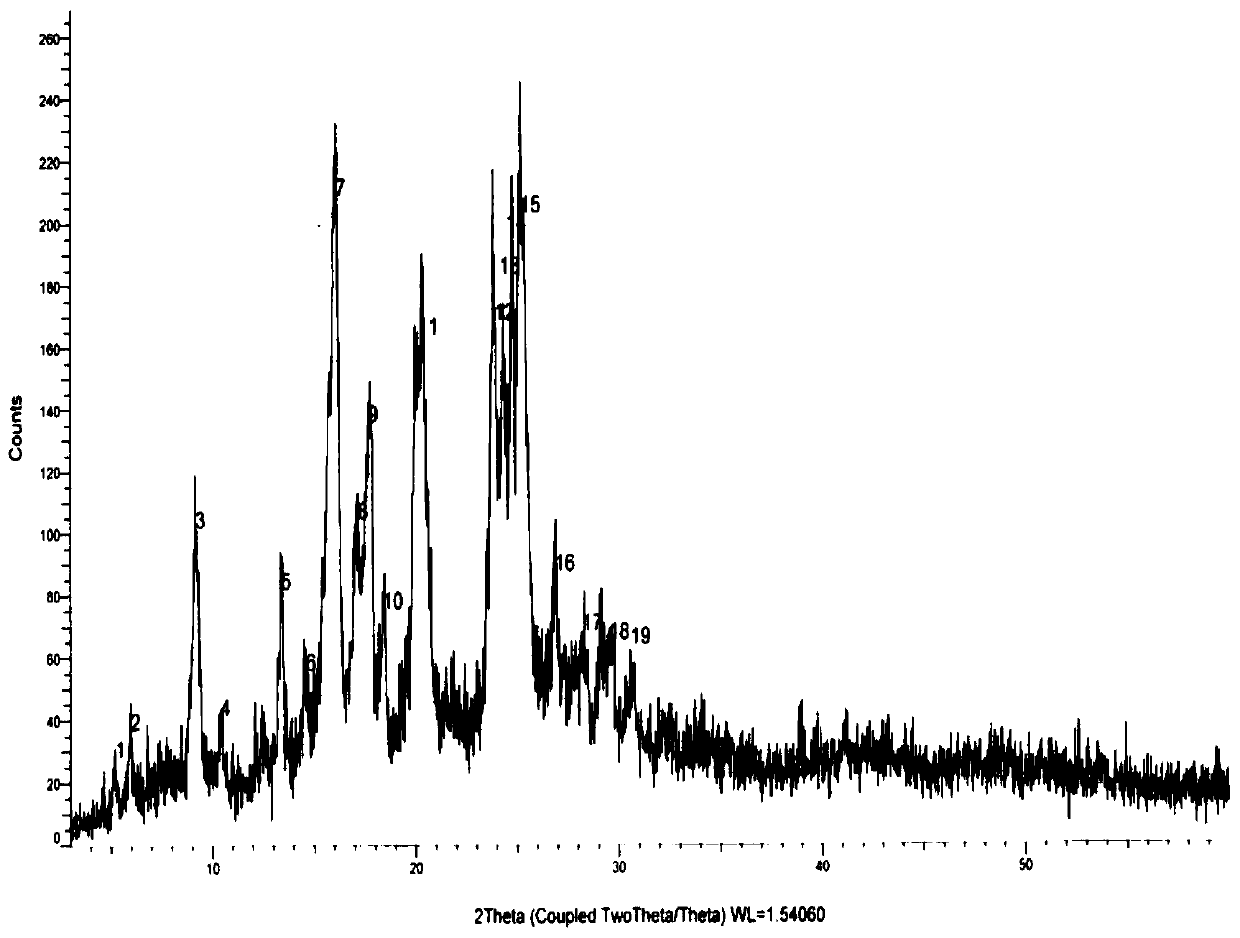
[0051] The XRD pattern data of embodiment 2-3 see Figure 1-2 , the particle size distribution figure of embodiment 2-1~2-4 is as follows Figure 5-8 shown.
[0052] In the process of adjusting the crystallization temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com