Application of chlorogenic acid in preparation of medicine or pharmaceutical composition for preventing or treating pain
A technology of composition and chlorogenic acid, which is applied in the direction of drug combination, drug delivery, antipyretics, etc., can solve the problems of three-step analgesic drug adverse reactions, achieve good clinical application prospects, have no toxic side effects, and relieve cancer pain Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Oral pharmaceutical preparation prescription of the present invention
[0046] 1. Prescription 1
[0047] Chlorogenic acid 1000g.
[0048] Preparation method: aseptically weigh chlorogenic acid, and aseptically pack into powder.
[0049] 2. Prescription II
[0050] Chlorogenic acid 1000g, filler 500g, binder 5g.
[0051] Preparation method: Weigh chlorogenic acid, fillers, and binders according to the prescription, granulate, granulate, and pack into granules.
[0052] 3. Prescription Three
[0053] Chlorogenic acid 1000g, filler 500g, binder 5g, lubricant 3g.
[0054] Preparation method: Weigh chlorogenic acid, filler and binder according to the prescription, granulate, granulate, add lubricant, compress into tablets to obtain tablets.
[0055] The above-mentioned filler is one or more of mannitol, lactose, starch, microcrystalline cellulose, and dextrin; the binder is sodium carboxymethyl cellulose, PVP; the lubricant is magnesium stearate, talcum p...
Embodiment 2
[0056] Embodiment 2 injection preparation prescription of the present invention
[0057] 1. Prescription 1
[0058] Chlorogenic acid 1000g.
[0059] Preparation method (1): Aseptically weigh chlorogenic acid according to the prescription, and aseptically subpackage it into powder injection.
[0060] Preparation method (2): Weigh chlorogenic acid according to the prescription, dissolve in water for injection, filter and sterilize, freeze-dry, and fill to obtain freeze-dried powder injection.
[0061] 2. Prescription II
[0062] Chlorogenic acid 1000g, scaffold agent 2667g, antioxidant 67g.
[0063] Preparation method: Weigh chlorogenic acid, scaffolding agent, and antioxidant according to the prescription, dissolve in water for injection, filter and sterilize, fill, and freeze-dry to obtain freeze-dried powder injection.
[0064] The above-mentioned support agent is mannitol, lactose, and glucose; the antioxidant is sodium bisulfite, vitamins, glutathione, and folic acid. ...
Embodiment 3
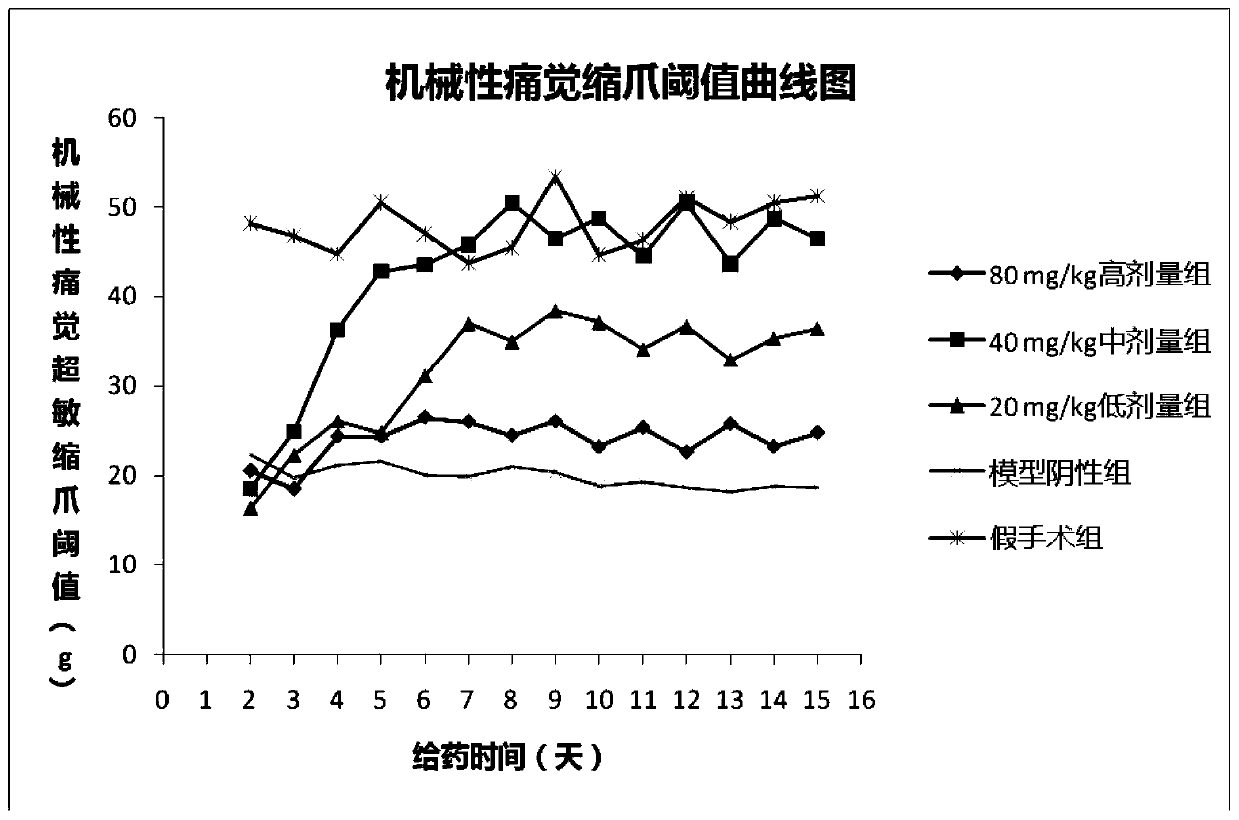
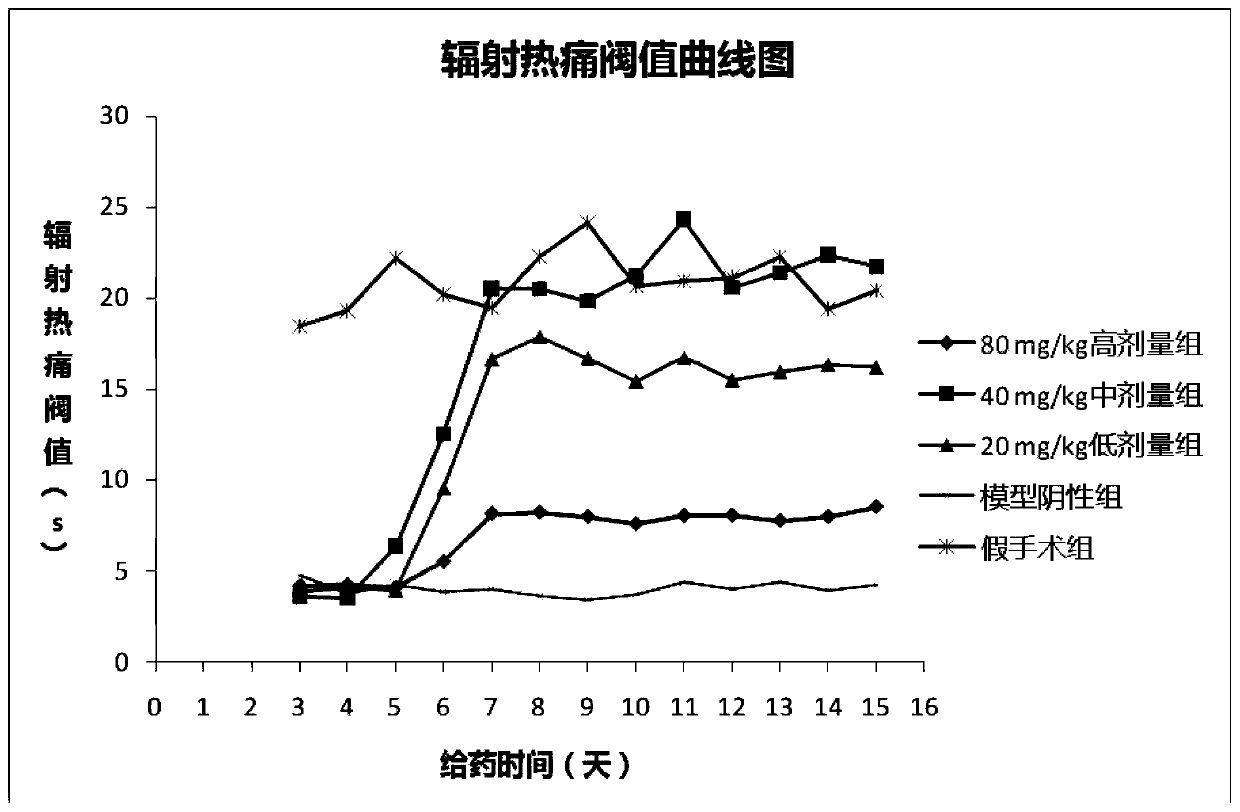
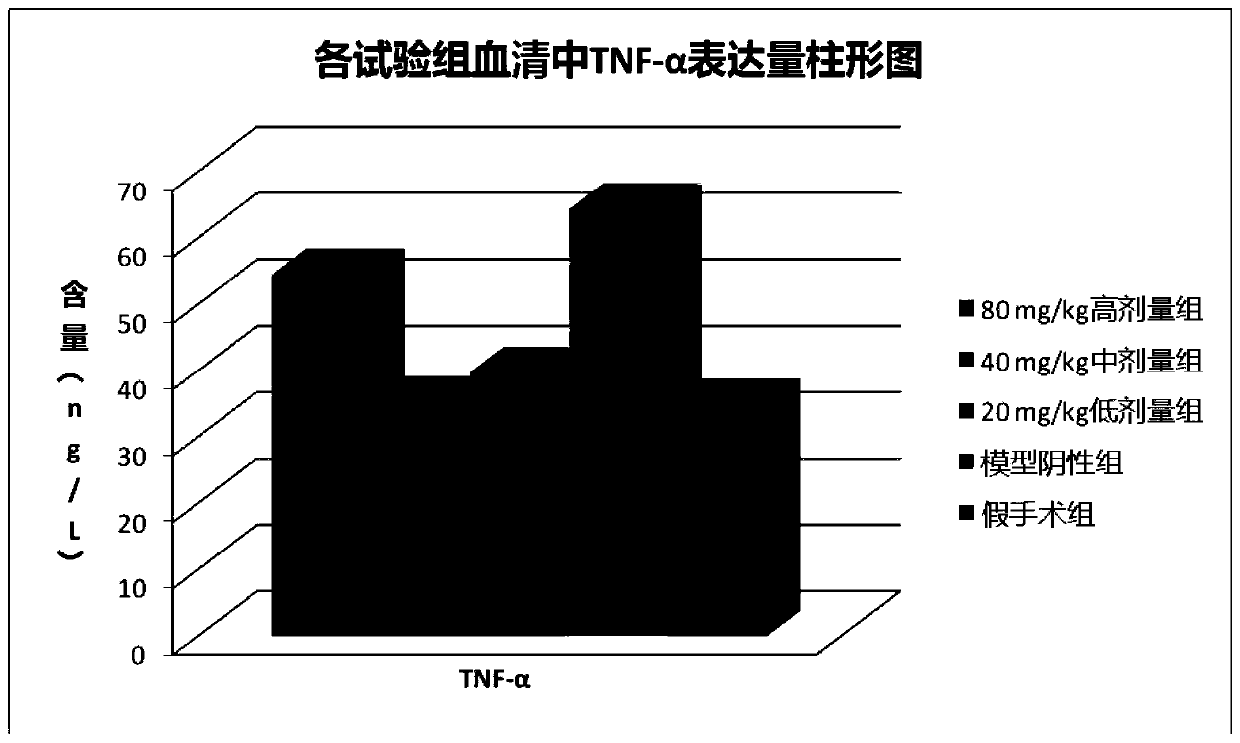
[0065] Example 3 Chlorogenic Acid Prevention or Treatment of Rat Bone Cancer Pain Animal Experiments
[0066] 1. Test material
[0067] 1.1 Animals
[0068] SD rats, female, weighing 180-200 g, were purchased from Chengdu Dashuo Experimental Animal Co., Ltd.
[0069] 1.2 Cell lines
[0070] Walker mouse breast cancer cell line was purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0071] 1.3 Drugs
[0072] Chlorogenic acid, batch number: 171101, content 99.83%, prepared by Sichuan Jiuzhang Biotechnology Co., Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com