Ultra-high performance liquid chromatography analysis method of clozapine related substances
An ultra-high performance liquid phase and chromatographic analysis technology, applied in the field of analysis of the purity of chemical drugs, can solve the problems of no relevant literature reports and long detection time, and achieve the effect of great positive progress and great practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: detection instrument and chromatographic conditions:
[0032] Ultra-Performance Liquid Chromatography: Waters UPLC AcQuity H-Class
[0033] Chromatographic column: Agilent Rapid Resolution HD column, specification 150×2.1mm, 1.8μm
[0034] Mobile phase A: methanol-buffer salt with a volume ratio of 25:75 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.40 with phosphoric acid);
[0035] Mobile phase B: methanol-buffer salt with a volume ratio of 90:10 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.40 with phosphoric acid);
[0036] Detection wavelength: 257nm;
[0037] Flow rate: 0.35ml / min
[0038] Column temperature: 35°C
[0039] Gradient elution program:
[0040]
[0041]
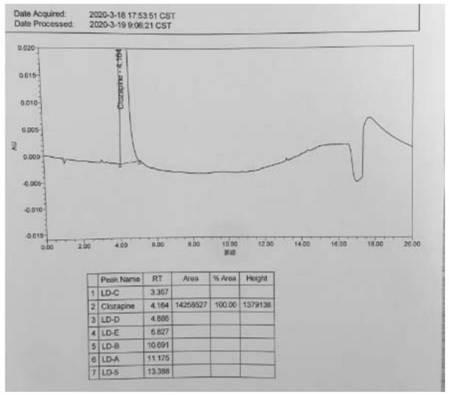
[0042] Step 1, preparation of the test solution: take clozapine 75mg in a 100ml volumetric flask, add 80ml of methanol and dilute to the mark with water, shake up as the test solution.
...
Embodiment 2
[0045] Embodiment 2: detection instrument and chromatographic conditions:
[0046] Ultra-Performance Liquid Chromatography: Waters UPLC AcQuity H-Class
[0047] Chromatographic column: Agilent Rapid Resolution HD column, specification 150×2.1mm, 1.8μm
[0048] Mobile phase A: methanol-buffer salt with a volume ratio of 25:75 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.35 with phosphoric acid);
[0049] Mobile phase B: methanol-buffer salt with a volume ratio of 90:10 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.35 with phosphoric acid);
[0050] Detection wavelength: 255nm;
[0051] Flow rate: 0.33ml / min
[0052] Column temperature: 33°C
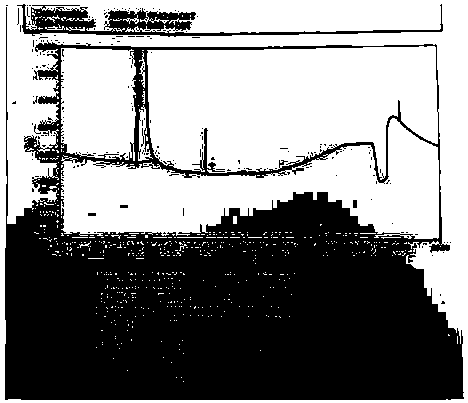
[0053] The test results are attached image 3 .
Embodiment 3
[0054] Embodiment 3: detection instrument and chromatographic conditions:
[0055] Ultra-Performance Liquid Chromatography: Waters UPLC AcQuity H-Class
[0056] Chromatographic column: Agilent Rapid Resolution HD column, specification 150×2.1mm, 1.8μm
[0057] Mobile phase A: methanol-buffer salt with a volume ratio of 25:75 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.45 with phosphoric acid);
[0058] Mobile phase B: methanol-buffer salt with a volume ratio of 90:10 (2.0g anhydrous potassium dihydrogen phosphate, add 1000ml water to dissolve, adjust pH 2.45 with phosphoric acid);
[0059] Detection wavelength: 259nm;
[0060] Flow rate: 0.37ml / min
[0061] Column temperature: 37°C
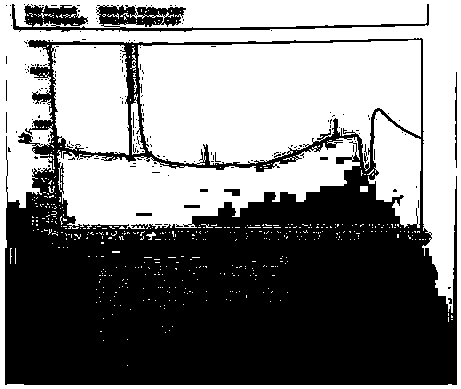
[0062] The test results are attached Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com