Artesunate succinylated derivative and preparation method and application thereof in medicines
A technology of artesunate and succinate, which is applied in the direction of organic chemical methods, drug combinations, and medical preparations containing active ingredients, etc., can solve the inconvenience of rescue medication for critically ill patients, the short half-life of artesunate, and the existence of liver and kidney toxicity To achieve the effect of improving the pathological changes of the pancreas, improving the state of the pancreas, and reducing serum inflammatory factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 artesunate
[0041] The reaction formula is as follows:
[0042]
[0043]1, the preparation of artesunate ethylene glycol ester (formula II compound)
[0044] Taking artesunate as raw material, add artesunate (1g, 2.6mmol), dicyclohexylcarbodiimide (DCC, 0.59g, 2.86mmol), 4-dimethylaminopyridine (DMAP , 0.1g, 0.78mmol), 5ml of dichloromethane, stirred and reacted at 0°C for 1h, added ethylene glycol (0.18g, 2.86mmol), reacted at room temperature for 10 hours, after the reaction was detected by TLC, filtered, and the filtrate was concentrated , and the residue was separated by column chromatography to obtain the target product, artesunate glycol ester (1 g, yield 90%, colorless oil).
[0045] Product H NMR spectrum: 1 H NMR (400MHz, CDCl 3 ):δ=6.79(d,J=8.01H),5.44(s,1H),4.30-4.18(m,2H),3.81(m,2H),2.78-2.68(m,4H),2.60-2.55( m, 1H), 2.42-2.34 (m, 1H), 2.06-1.24 (m, 16H), 0.96 (d, J=4.03H), 0.86 (d, J=4.03H) ppm.
[0046] 2, the prep...
Embodiment 2
[0049] Example 2 Artesunate Treatment of Sepsis Action Test
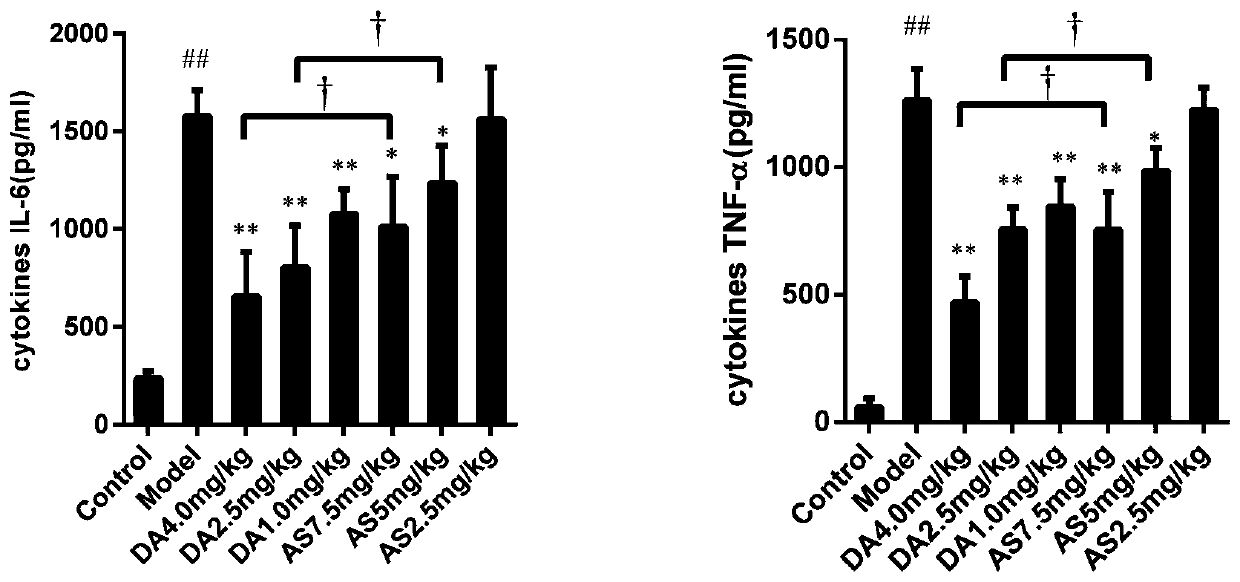
[0050] First, establish a sepsis mouse model of mice challenged with sublethal doses of heat-inactivated Escherichia coli, and observe the therapeutic effects of artesunate (AS) and artesunate (DA) alone on the model, whether Improve the inflammatory response and reduce the levels of inflammatory factors in sepsis model animals.
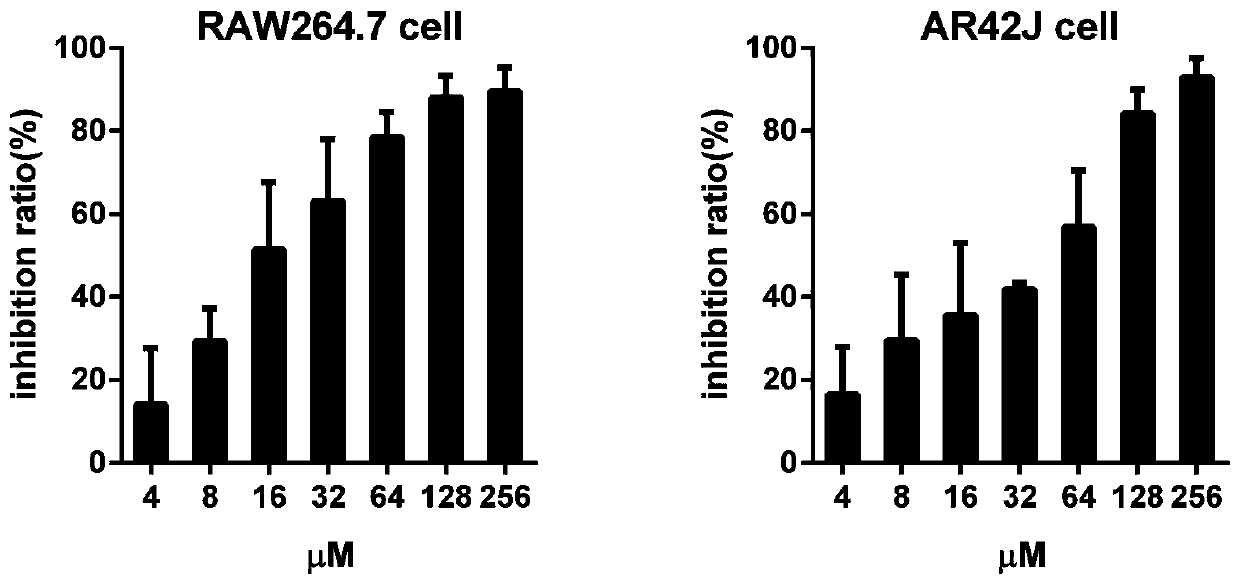
[0051] LD50 50 For (618.24mg / kg); The cytotoxicity test result of DA sees ( figure 1 ): mouse monocyte-macrophage (RAW264.7 cell), 10% inhibition rate of DA (IC 10 ) was 2.03 μM, while IC in rat pancreatic acinar cells (AR42J cells) 10 2.84μM, with IC 10 The converted safe dosage for mice is about 4.2 mg / kg. The clinical dosage of artesunate AS is 60mg / d, and the equivalent dosage used in mice is about 7.5mg / kg converted according to the human-mouse body surface area dosage formula.
[0052] A mouse model of sepsis was established by tail vein injection of sublethal doses of heat-kil...
Embodiment 3
[0056] Example 3 Effect test of artesunate in treating acute pancreatitis
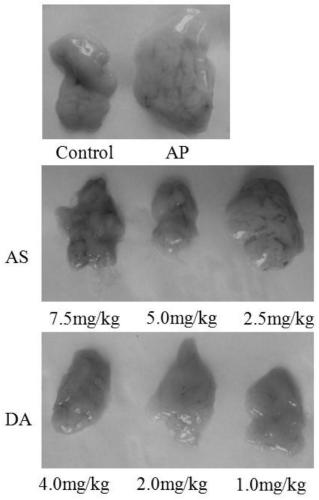
[0057] A mouse model of acute pancreatitis induced by cerulein combined with lipopolysaccharide, to observe the therapeutic effects of artesunate (AS) and artesunate (DA) alone on the model, whether it can improve the pancreas inflammatory response of acute pancreatitis model animals , reducing enzyme activity.
[0058] A mouse model of acute pancreatitis was established using cerulein combined with LPS (lipopolysaccharide). The experimental mice were fasted for 12 h before model making, and were not allowed to drink water. 80 mice, half male and half male, were randomly divided into 8 groups with 10 mice in each group. Acute pancreatitis mouse model was prepared by injecting LPS (10 mg / kg) for the last time with 100 μg / kg cerulein for 6 consecutive times with an interval of 1 hour. The groups were divided into: three doses of artesunate treatment group (DA4.0 mg / kg, DA2.0 mg / kg group, DA1.0 mg / kg g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com