Environment-friendly polyisocyanate synthesis method suitable for industrial production
A polyisocyanate and dihydrooxime technology, applied in the field of isocyanate, can solve the problems of slow reaction rate, high price, and many side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In a 2L three-necked flask, dissolve 0.135mol of ethyl terephthalate in 400ml of methanol at room temperature; dissolve 0.81mol of sodium hydroxide in 90ml of distilled water, and dissolve 0.81mol of hydroxylamine hydrochloride in 60ml of distilled water, and mix Two alkaline solutions were added dropwise to the terephthalate solution at 5°C, then the reactant was stirred at 55°C for 72 hours; the above mixture was neutralized to pH with 700ml of 5% hydrochloric acid to 6-7, then filter the solution, and stir the solid obtained in 1L boiling methanol; afterward, the mixture is filtered to obtain white solid terephthalic acid, the yield of the reaction is 92%, and the purity of the product can Reached 97%.
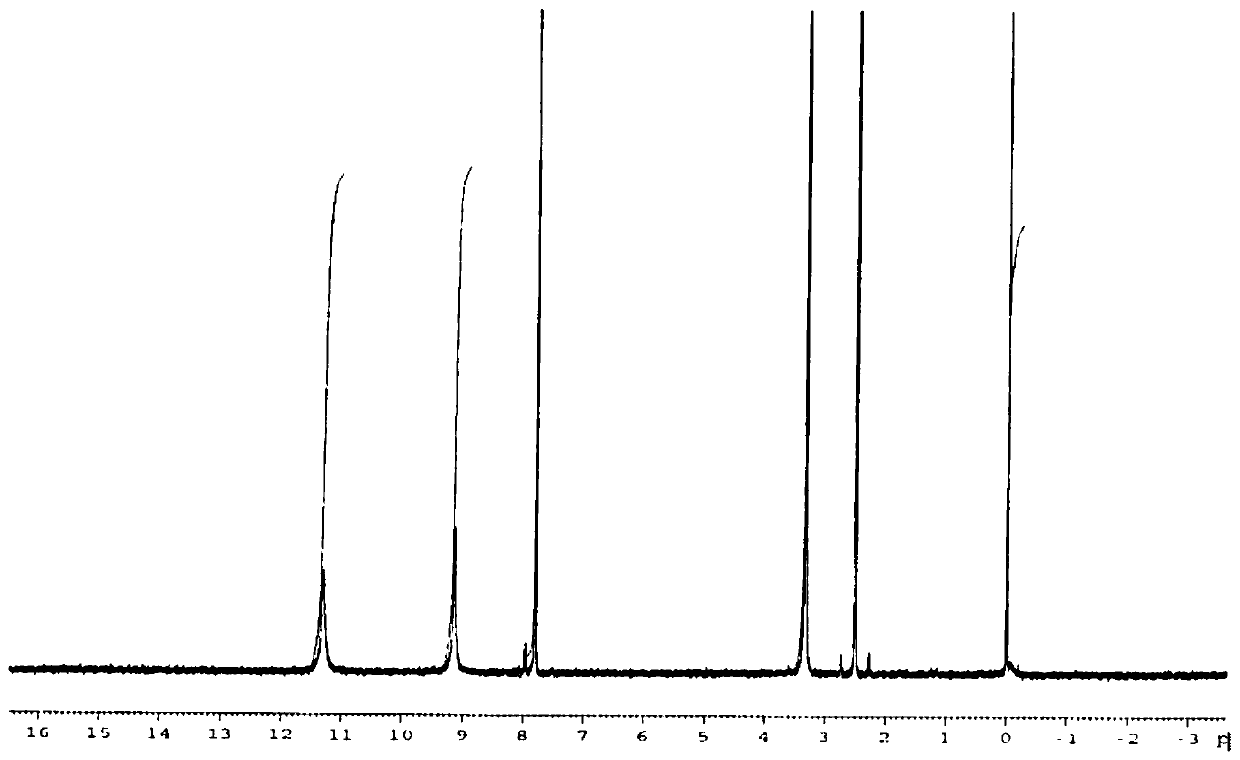
[0055] figure 1 Be the H-NMR spectrogram of p-phthalic dihydroxamic acid in the embodiment of the present invention 1; By figure 1 It can be seen that the single peak 7.8ppm corresponds to 4H C-H aromatic compound, the single peak 9.1ppm corresponds to 2HO-H, and t...
Embodiment 2
[0059] In a 2L three-necked flask, 0.135mol of ethyl toluate was dissolved in 400ml of methanol at room temperature; 0.81mol of sodium hydroxide was dissolved in 90ml of distilled water, and hydroxylamine hydrochloride was dissolved in 60ml of distilled water. solution, and added dropwise to the terephthalate solution at 5°C, and then the reactant was stirred at 55°C for 72 hours; the above mixture was neutralized to pH 6-7 with 700ml of 5% hydrochloric acid , Then the solution was filtered, and the obtained solid was stirred in 1L of boiling methanol; after that, the mixture was filtered to obtain a white solid dihydroxamic acid, the yield of the reaction was 93%, and the purity of the product reached 97%.
[0060] Weigh a certain mass of the white solid dihydroxamic acid obtained above, add 2 times the equivalent of 4-nitrobenzenesulfonyl chloride and 4 times the equivalent of triethylamine, all dissolved in excess tetrahydrofuran, at 0-25 After fully stirring at ℃ for 6 hou...
Embodiment 3
[0065] In a 2L three-necked flask, 0.135mol of ethyl diphenylmethane dicarboxylate was dissolved in 400ml of methanol at room temperature; 0.81mol of sodium hydroxide was dissolved in 90ml of distilled water, and hydroxylamine hydrochloride was dissolved in 60ml of distilled water. An alkaline solution was added dropwise to the terephthalate solution at 5°C, and the reactant was stirred at 55°C for 72 hours; the above mixture was neutralized to pH 6 with 700ml of 5% hydrochloric acid -7, then filter the solution, and stir the obtained solid in 1 L of boiling methanol. Afterwards, the mixture was filtered to obtain white solid dihydroxamic acid, the yield of the reaction was 92%, and the purity of the product could reach 98%.
[0066] Weigh a certain mass of the white solid dihydroxamic acid obtained above, add 2 times the equivalent of 4-nitrobenzenesulfonyl chloride and 4 times the equivalent of triethylamine, all dissolved in excess tetrahydrofuran, at 0-25 After fully stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com