GIP/GLP-1 analogue
An analog, the technology of reparatide, which is applied in the field of tireparatide analogs, can solve the problems of not being able to achieve full-effect blood sugar control and weight loss, and achieve the effects of stable properties, weight reduction, and hypoglycemic weight loss.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 compound 1
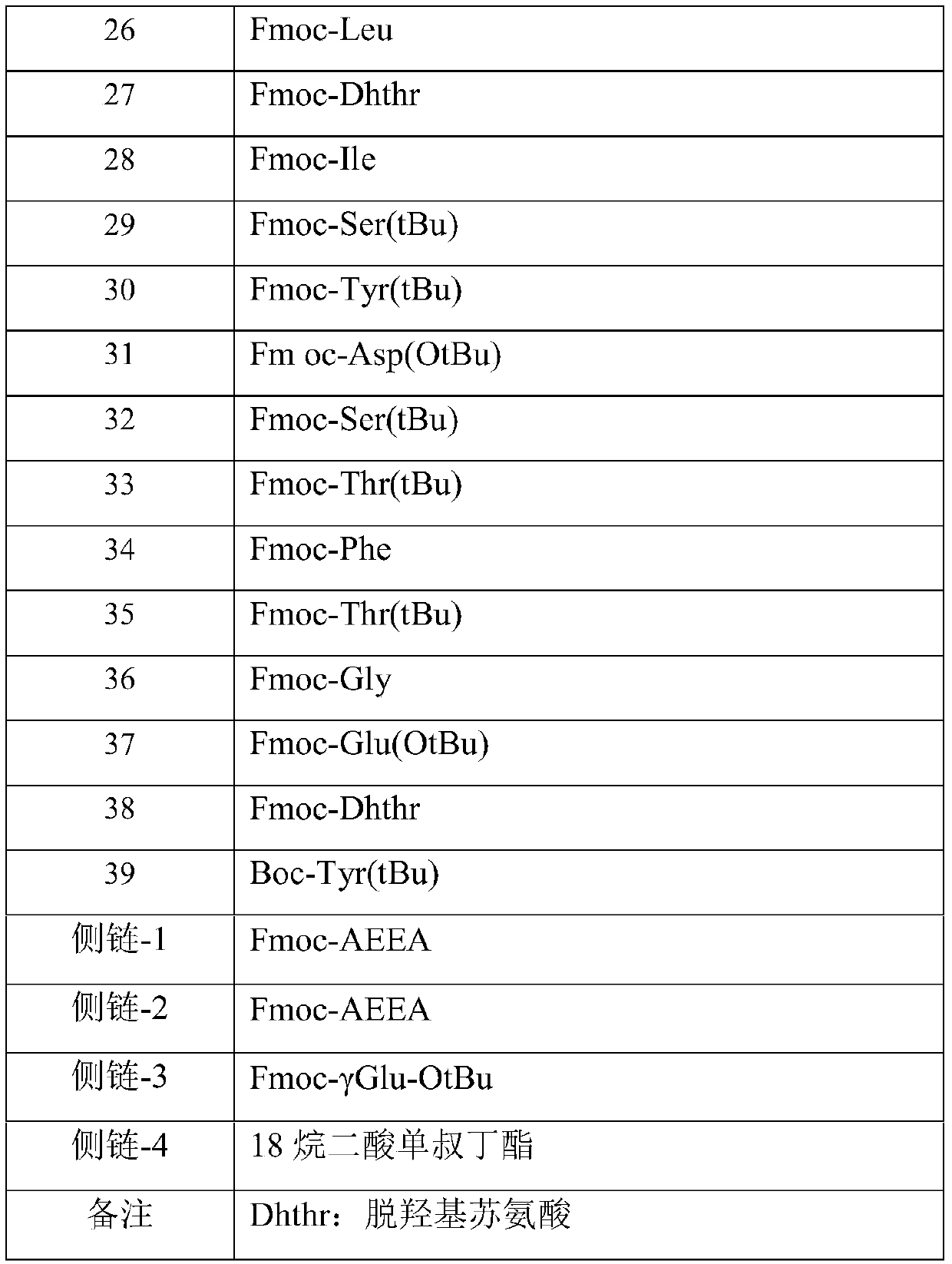
[0033] Tyr-Dhthr-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhthr-Leu-Asp-Lys-Ile-Ala-Gln-Lys (AEEA-AEEA-γGlu-18 alkanedioic acid )-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH 2
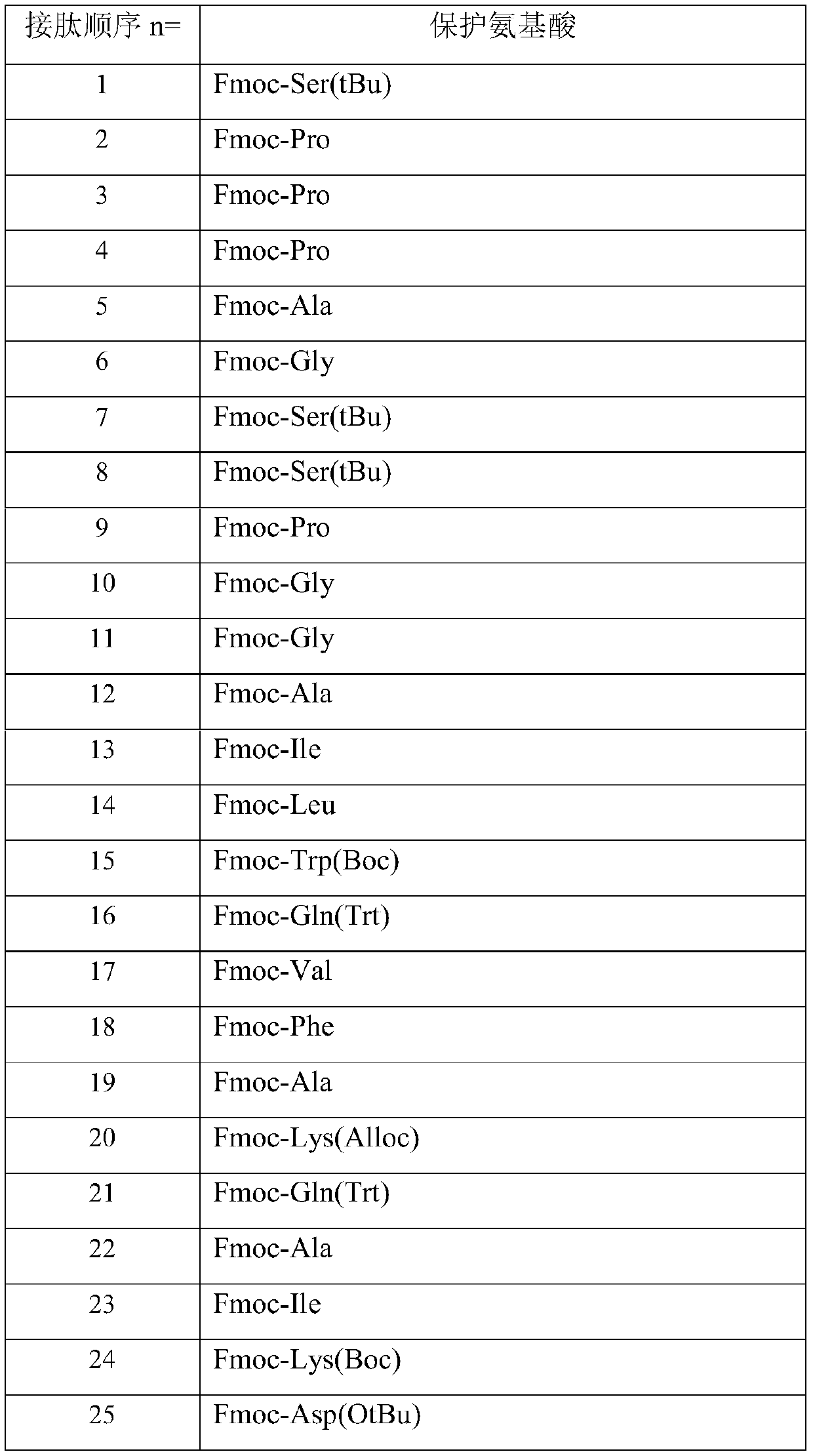
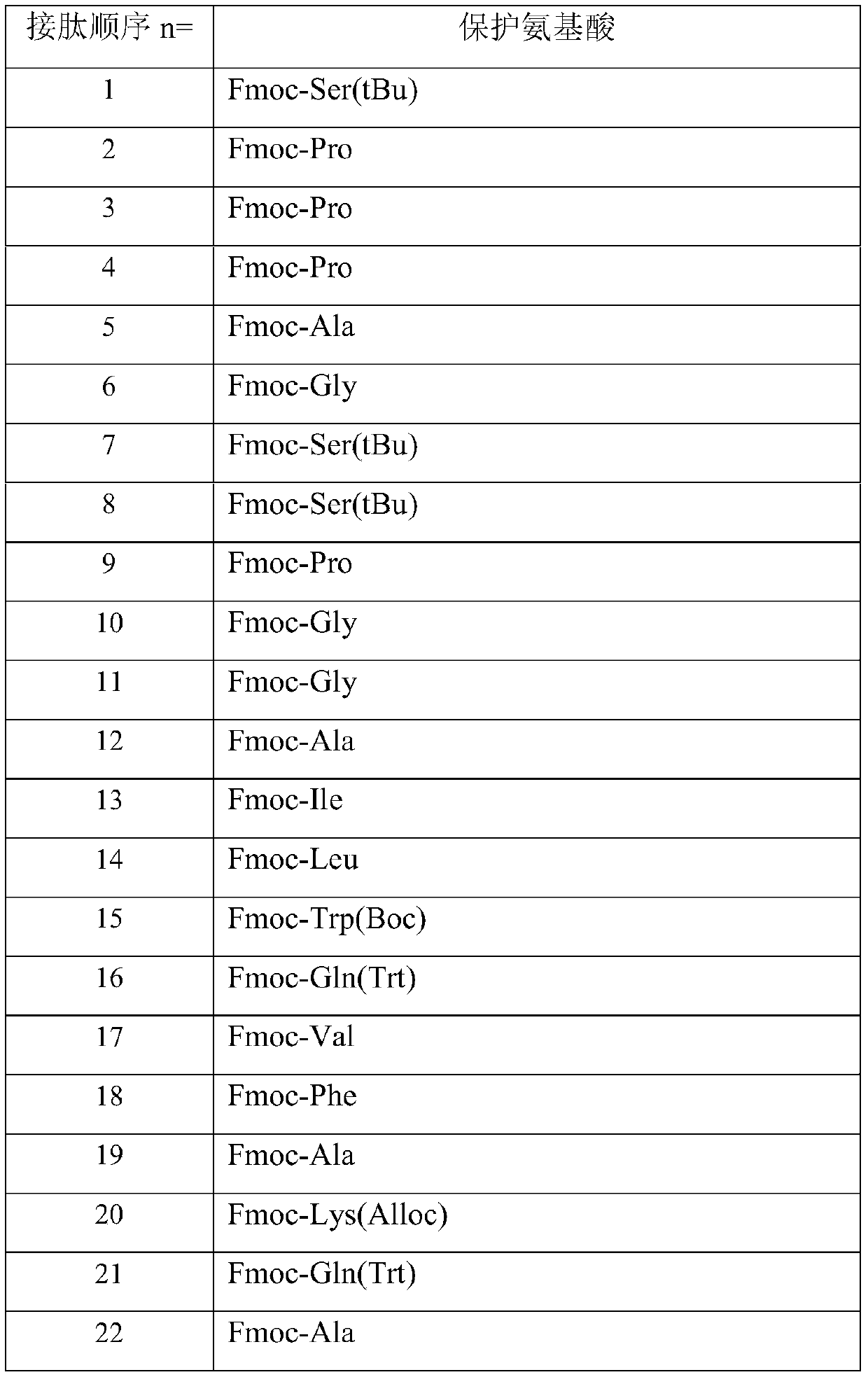
[0034] The preparation method includes: preparing the peptide resin by solid-phase polypeptide synthesis method, acid hydrolyzing the peptide resin to obtain a crude product, and finally purifying the crude product to obtain a pure product; wherein the step of preparing the peptide resin by the solid-phase polypeptide synthesis method is to pass solid-phase The phase-coupled synthesis method sequentially inserts the corresponding protected amino acids or fragments in the following sequences to prepare peptide resins:
[0035] In the above preparation method, the amount of the Fmoc-protected amino acid or protected amino acid fragment is 1.2-6 times of the total moles of the resin fed; preferably 2.5-3.5 times.
[0...
Embodiment 2
[0069] The preparation of embodiment 2 compound 2
[0070] Tyr-Dhval-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhval-Leu-Asp-Lys-Ile-Ala-Gln-Lys (AEEA-AEEA-γ-Glu-18 alkane Diacid)-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH 2
[0071] The preparation method is the same as in Example 1, and the protected amino acids used are as follows:
[0072]
[0073]
[0074] 6.1 g of pure product was obtained, the purity was 96.9%, and the total yield was 12.5%. Molecular weight 4809.5 (100% M+H).
Embodiment 3
[0075] The preparation of embodiment 3 compound 3
[0076] Tyr-Dhthr-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhthr-Leu-Asp-Lys-Ile-Ala-Gln-Lys(PEG 5 CH 2 CO-γGlu-18 alkanedioic acid)-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH 2
[0077] The preparation method is the same as in Example 1, and the protected amino acids used are as follows:
[0078]
[0079]
[0080] 7.4 g of pure product was obtained, the purity was 96.3%, and the total yield was 15.3%. Molecular weight 4768.4 (100% M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com