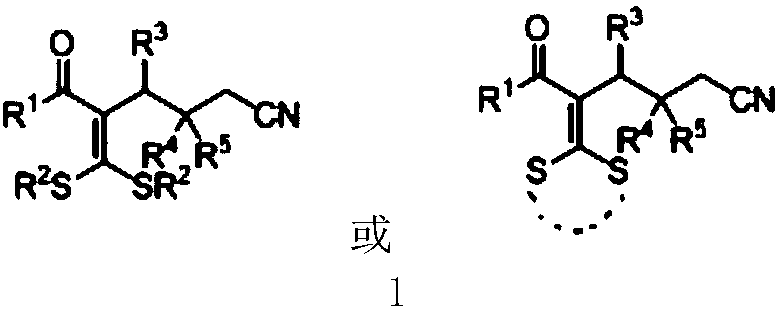
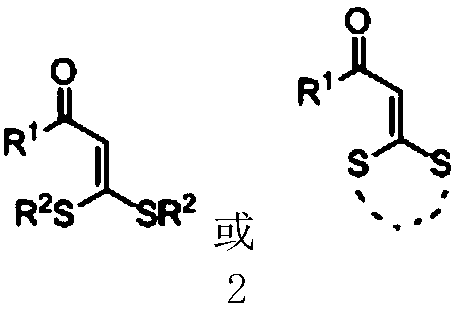
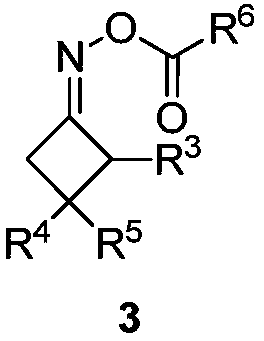
Cyanoalkyl substituted tetra-substituted olefin derivatives and synthesis thereof
A tetra-substituted, cyanoalkyl technology, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of high cost and poor atom economy, and achieve the effects of simple operation, low cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] In a 25mL schlenk tube, add 3,3-dialkylthio-2-propene-1-one compound 2a (0.3mmol), cyclobutanone oxime ester 3a (0.6mmol), iron trichloride ( 10mol%) and 1.5 mL of benzotrifluoride at 110°C for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=50:1) to obtain the target product 1a (69mg, 79% yield). The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
[0034] Compound characterization data
[0035] Cyano-substituted tetra-substituted olefin derivative (1a), yellow liquid. 1 H NMR(400MHz, CDCl 3 )δ7.86,7.57and 7.47(m each,2:1:2H,aromatic CH),2.79(dd,J=8.6and 6.9Hz,2H,(C=O)CCH 2 ),2.39(m,5H,CH 2 CN and SMe),2.07(s,3H,SMe),1.84(m,2H,CH 2 CH 2 CN). 13 C{ 1 H)NMR(100MHz,CDCl 3 )δ196.59(Cq,C=O),144.7,136.8and 136.5(Cq),133.5,129.0and128.8(arom...
Embodiment 2
[0037]
[0038] In a 25mL schlenk tube, add 3,3-dialkylthio-2-propene-1-one compound 2b (0.3mmol), cyclobutanone oxime ester 3a (0.6mmol), iron trichloride ( 10mol%) and 1.5 mL of benzotrifluoride at 110°C for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=50:1) to obtain the target product 1b (73mg, yield 76%). The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
[0039] Compound characterization data
[0040] Cyano-substituted tetra-substituted olefin derivative (1b), yellow liquid. 1 H NMR(400MHz, CDCl 3 )δ7.84, 7.57 and 7.45 (m each, 2:1: 2H, aromatic CH), 2.82 (m, 4H, SCH 2 and(C=O)CCH 2 ), 2.62(q,J=7.4Hz,2H,SCH 2 ), 2.38(t,J=7.2Hz,2H,CH 2 CN),1.84(m,2H,CH 2 CH 2 CN),1.30(t,J=7.3Hz,3H,CH 2 CH 3 ),1.02(t,J=7.4Hz,3H,CH 2 CH 3 ). 13 C{ 1 H)NMR(100MHz,...
Embodiment 3
[0042]
[0043] The reaction steps and operations are the same as in Example 1, and the difference from Example 1 is that the 3,3-dialkylthio-2-propene-1-one compound is 2c. The reaction was stopped, and the target product 1c (66 mg, yield 76%) was obtained after post-treatment. The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
[0044] Cyano-substituted tetra-substituted olefin derivative (1c), yellow liquid. 1 H NMR(400MHz, CDCl 3 )δ7.44 (m, 5H, aromatic CH), 3.38 (m, 4H, SCH 2 CH 2 S), 2.73(dd,J=8.6and 6.9Hz,2H,(C=O)CCH 2 ),2.21(t,J=7.2Hz,2H,CH 2 CN),1.75(m,2H,CH 2 CH 2 CN). 13 C{ 1 H)NMR(100MHz,CDCl 3 )δ193.6(C q ,C=O),162.3,139.4and 122.9(C q ),130.9,128.4and 127.5(aromatic CH),119.2(CN),39.1,36.5,33.8,24.2and 16.8(CH 2 ).HRMS Calcd for C 15 H 15 NOS 2 [M+H] + :290.0673; Found:290.0672.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com