Tumor self-targeting multilevel response type mesoporous silicon drug delivery system and preparation method thereof
A drug delivery system and tumor-targeting technology, which is applied in the field of tumor self-targeting multi-level responsive mesoporous silicon drug delivery system and its preparation, can solve the problem of discounting anti-tumor treatment effects, premature drug release, and harming normal tissues, etc. problems, to achieve the effects of reduced damage, improved endocytosis, stable and controllable drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation and Characterization of Mesoporous Silicon Drug Delivery System with Temperature and pH Dual Response and Tumor Targeting
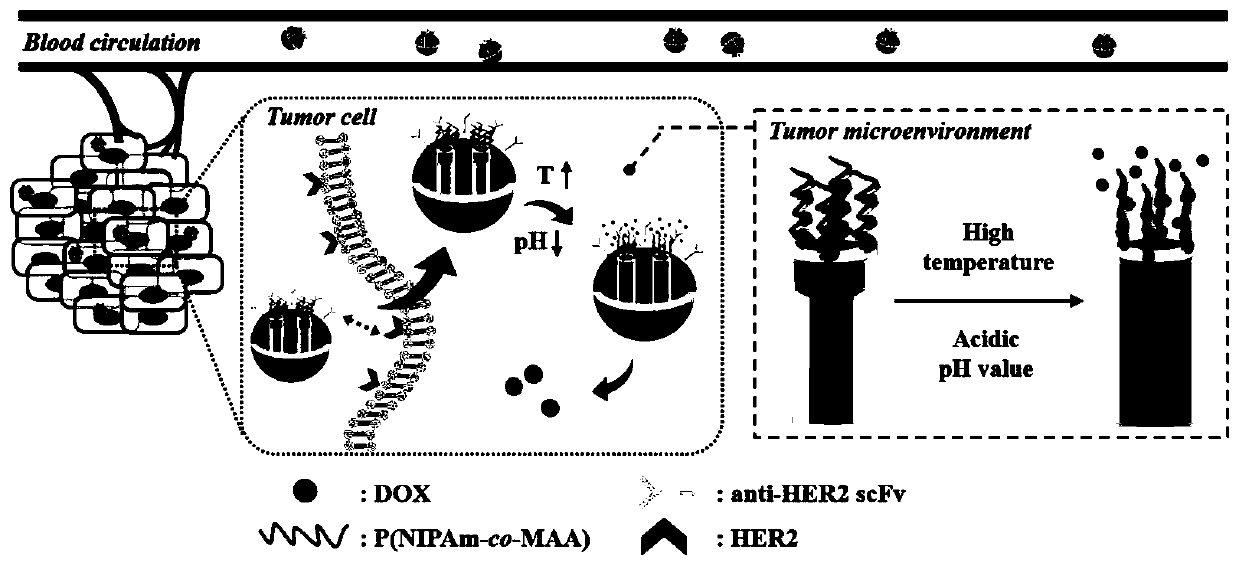
[0043] Such as Figure 1A As shown, the present invention provides a highly biocompatible tumor-targeting intelligent multi-response mesoporous silicon drug delivery system and its preparation method, including: synthesizing and preparing mesoporous silicon nanoparticles, loading anti-tumor drugs after silylation, Subsequently, N-isopropylacrylamide (NIPAm) and methacrylic acid (MAA) copolymers were covalently modified, and anti-HER2 single-chain antibodies were grafted to obtain nanoparticles with tumor targeting and dual responsiveness to temperature and pH.
[0044] Among them, by modifying the silane coupling agent MPS on the surface of the synthesized mesoporous silicon nanoparticles, anti-tumor drugs are loaded in the pores of the mesoporous silicon nanoparticles; the temperature and pH double response layer is modified ...
Embodiment 2
[0053] Example 2: Cytocompatibility and Hemocompatibility
[0054] The experimental steps are as follows:
[0055] Human breast cancer cells (SK-BR-3) with high expression of HER2 receptors were subcultured, spread on 96-well plates at 5,000 / well, and after 24 hours of incubation, MSN or MSN-pNIPAm / MAA-HER2 were added respectively to make The final particle concentration is 0, 5, 10, 50, 100, 200, 500 μg / mL, and continue to incubate for 24 hours;
[0056] Subsequently, remove the orifice plate and add tetramethylazolidine (MTT) to each well, and then put it in 37°C, 5% CO 2 Cultivate in the incubator for 4 hours;
[0057] Finally, take out the orifice plate and absorb the supernatant, add dimethyl sulfoxide (DMSO), shake for 10 minutes, measure the absorbance of each well with a microplate reader, record the relative cell viability when the concentration is 0 μg / mL as 100%.
[0058] Experimental results, such as Figure 3A As shown, at the same particle concentration, the ...
Embodiment 3
[0064] Example 3: Temperature and pH dual response drug release
[0065] Experimental steps:
[0066] Prepare pH 7.4 phosphate buffer (simulating human blood pH) and pH 5.0 phosphate buffer (simulating intracellular pH of human tumor tissue);
[0067] Disperse 2mg of DOX@MSN-pNIPAm / MAA-HER2 in phosphate buffer and react at 37°C and 25°C;
[0068] The supernatant was taken at a certain time interval, detected by a UV-visible spectrophotometer, and the cumulative release curve was drawn according to the ratio of the cumulative released drug amount to the total loaded drug amount at each time point.
[0069] Such as Figure 4 As shown, when high temperature (41°C) and acidic (pH 5.0) stimuli were used to jointly trigger drug release, the resulting drug release was faster and the release amount was higher than that of a single stimulus condition, indicating that the modified mesoporous Silicon nanoparticles have dual-response drug release behavior of temperature and pH, and hav...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com