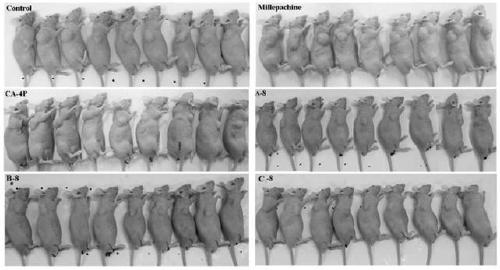
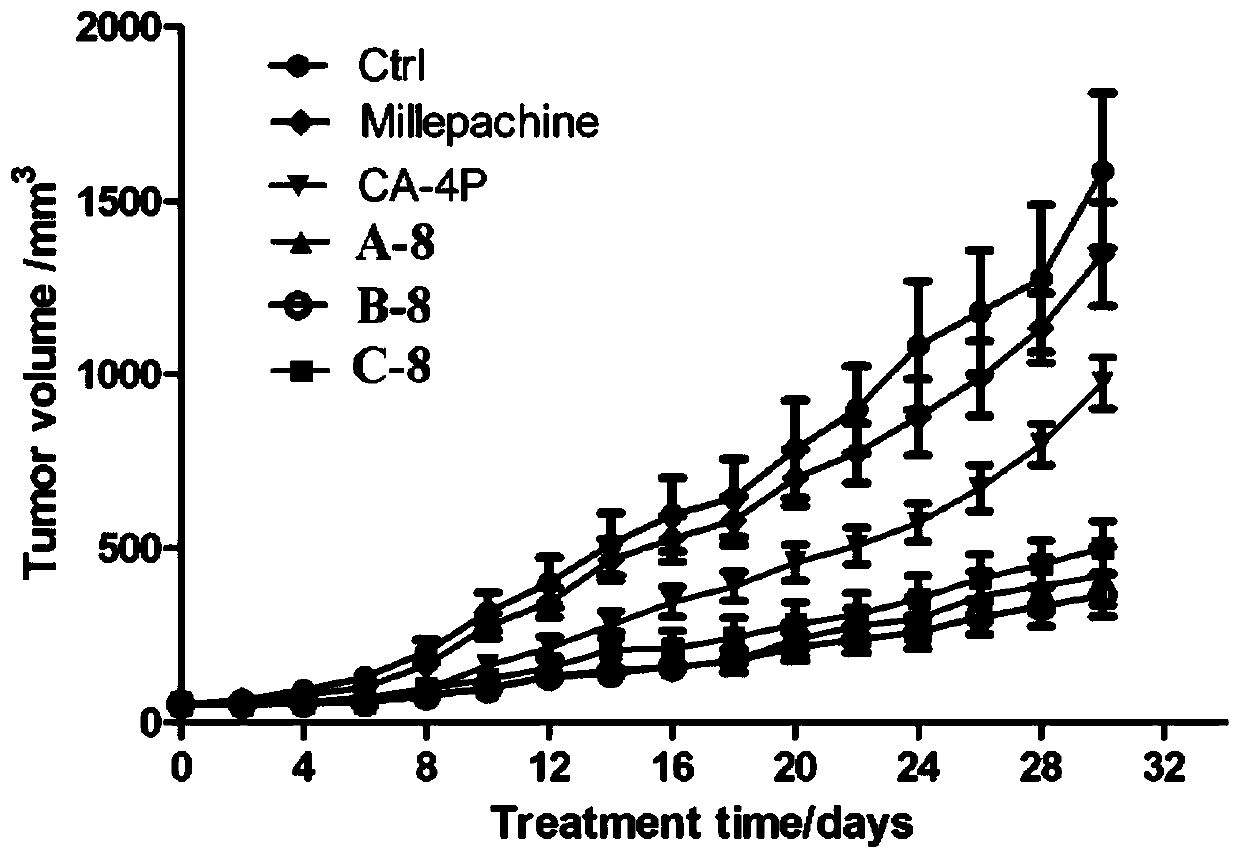
Millepachine-CA-4 derivative as well as preparation method and application thereof
A technology of derivatives and preparation steps, applied in the field of medicinal chemistry, can solve the problems of CA-4 instability, easy photolysis, poor water solubility, etc., and achieve the effect of improved anti-tumor activity, less toxic and side effects, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1. Preparation of compound A-1
[0056]
[0057] (1) Intermediate 3: Preparation of 5-methoxy-2,2-dimethyl-2H-pyran-benzaldehyde.
[0058]
[0059] The commercial raw material 1 (1mmol) was dissolved in 10mL acetonitrile, and DBU (2mmol), 3-chloro-3-methyl-1-butyne (1.5mmol), chlorinated ketone ( 0.1 mmol), after stirring for 8 h, dilute hydrochloric acid was added to adjust the pH=2, and then ethyl acetate was added for extraction. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was separated by column chromatography (petroleum ether: ethyl acetate, 10:1) to obtain intermediate 2.
[0060] Intermediate 2 (1 mmol) was dissolved in 10 mL of pyridine, reacted at 70°C for 10 hours, and the solvent was removed under reduced pressure to obtain a crude product, which was separated by column chromatography (petroleum ether: ethyl acetate...
Embodiment 2
[0074] Embodiment 2. Preparation of Compound A-2
[0075]
[0076] Compound A-1 (2 mmol) was dissolved in dry methanol, sodium methoxide (6 mmol) was added under stirring at room temperature, and the reaction was refluxed for 8 hours. After the reaction, the system was cooled to room temperature. Ethyl acetate was added for extraction, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography to obtain the target compound A-2. White solid, 73% yield. 1 HNMR (400MHz, Chloroform-d) δ9.24(s,1H),7.57(s,2H),7.27(d,J=7.5Hz,1H),6.85(d,J=7.5,1H),6.66(d ,J=1.9Hz,1H),6.47(d,J=7.5Hz,1H),5.23(d,J=8.8Hz,1H),4.10(d,J=4.9Hz,2H),3.95(s,3H ),3.87(s,3H),2.87(t,J=5.0Hz,1H),1.44(s,6H). 13 C NMR(101MHz,Chloroform-d)δ197.80,171.54,159.04,155.17,152.77,140.60,134.73,127.03,126.10,122.91,117.66,115.30,111.37,108.73,107.61,106.65,77.48,61.00,56.27,55.93,27.87...
Embodiment 3
[0080] Embodiment 3. Preparation of compound A-3
[0081]
[0082] Compound A-1 (2mmol) was dissolved in ethanol (15mL), and 25% NH 3 ·H 2 O (8 mmol), react at room temperature for 3 hours. After the reaction, the system was extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography to obtain the target compound A-3. White solid, 65% yield. 1 HNMR (400MHz, Chloroform-d) δ9.26(s, 1H), 7.92(s, 2H), 7.59(d, J=7.5Hz, 1H), 7.26(d, J=7.5Hz, 1H), 6.72( d,J=9.5Hz,1H),6.47(d,J=8.6Hz,1H),5.27(d,J=10.8Hz,1H),3.85(s,2H),3.83(s,3H),3.81( s,3H),1.54(s,2H),1.47(s,6H). 13 C NMR(101MHz,Chloroform-d)δ194.7,168.48,159.04,155.45,150.13,140.93,132.53,128.36,127.71,123.34,117.66,116.75,111.37,109.43,106.65,103.37,76.48,56.01,55.56,44.32,27.82 .
[0083] The synthesis method of compound B-3 and C-3 is the same as that of com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average volume | aaaaa | aaaaa |
| Average volume | aaaaa | aaaaa |
| Average weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com