Preparation method and application of clethodim intermediate
A technology of clethodim and intermediates, applied in the field of clethodim preparation, can solve the problems of affecting the yield and product quality, increasing the burden of wastewater treatment, and high ammonia nitrogen content, so as to facilitate separation and purification, increase the burden of wastewater treatment, and improve product yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
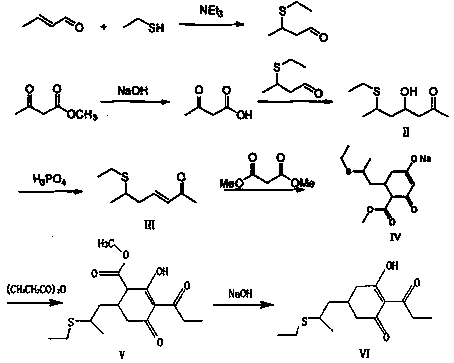
[0044] 1. Refer to the method of patent 201710535450.4 to prepare 6-ethylthio-3-hepten-2-one toluene solution.
[0045] 2. Put 45g of sodium methoxide methanol solution with a mass concentration of 30% into a 500ml three-necked flask, distill off the methanol, then add 250g of toluene and 40g of diethyl malonate, stir for 1h, and then dropwise add 30wt% of 6-Ethylthio-3-hepten-2-one toluene solution 137g, dropwise time is 1h, after dropping, keep warm at 40°C for 1h for cyclization reaction, after reaction, heat up to 70°C for dealcoholization, and add 120g of toluene at the same time, After dealcoholization is finished, obtain the mixture of 240g compound A and toluene, compound A structural formula is as follows:
[0046]
[0047] 3. Filter the mixture of compound A and toluene obtained above to remove impurities, put the obtained compound A into a three-necked flask containing 200g of toluene, start stirring, raise the temperature to 85-95°C, and then add H -ZSM-5 molec...
Embodiment 2
[0050] 1. Refer to the method of patent 201710535450.4 to prepare 6-ethylthio-3-hepten-2-one toluene solution.
[0051] 2. Put 45g of sodium methoxide methanol solution with a mass concentration of 30% into a 500ml three-necked flask, distill off the methanol, then add 250g of toluene and 33g of dimethyl malonate, and dropwise add 30wt% 6- Ethylthio-3-hepten-2-one toluene solution 137g, dropwise time is 1h, after dropping, keep warm at 40°C for 1h for cyclization reaction, heat up to 70°C after reaction to remove solvent, add 120g of toluene at the same time, remove After alcohol finishes, obtain the mixture of 225g compound A and toluene, compound A structural formula is as follows:
[0052]
[0053] 3. Filter the mixture of compound A and toluene obtained above to remove impurities, put the obtained compound A into a three-necked flask containing 200g of toluene, start stirring, and control the temperature at 105-110°C, and then add ZSM- 5. Molecular sieve catalyst 5.0g ...
Embodiment 3
[0056] 1. Refer to the method of patent 201710535450.4 to prepare 6-ethylthio-3-hepten-2-one toluene solution.
[0057] 2. Put 45g of sodium methoxide methanol solution with a mass concentration of 30% into a 500ml three-necked flask, distill off the methanol, then add 250g of toluene and 33g of dimethyl malonate, and dropwise add 30wt% 6- Ethylthio-3-hepten-2-one toluene solution 137g, dropwise time is 1h, after dropping, keep warm at 40°C for 1h for cyclization reaction, heat up to 70°C after reaction to remove solvent, supplement 120g of toluene at the same time, remove After alcohol finishes, obtain the mixture of 225g compound A and toluene, compound A structural formula is as follows:
[0058]
[0059] 3. Filter the mixture of compound A and toluene obtained above to remove impurities, put the obtained compound A into a three-necked flask containing 200g of toluene, start stirring, raise the temperature to 50-60°C, and then add H - 2.5g of ZSM-5 molecular sieve catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com