Device and method for preparing vitamin A acetate by multistage continuous tandem reaction extraction
A technology of series reaction and extraction device, applied in the direction of organic chemistry, etc., can solve the problems of increasing the synthesis yield of vitamin A acetate, high labor intensity, and increasing side reactions, etc., so as to reduce the labor intensity of process operation and improve industrial production. level, the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
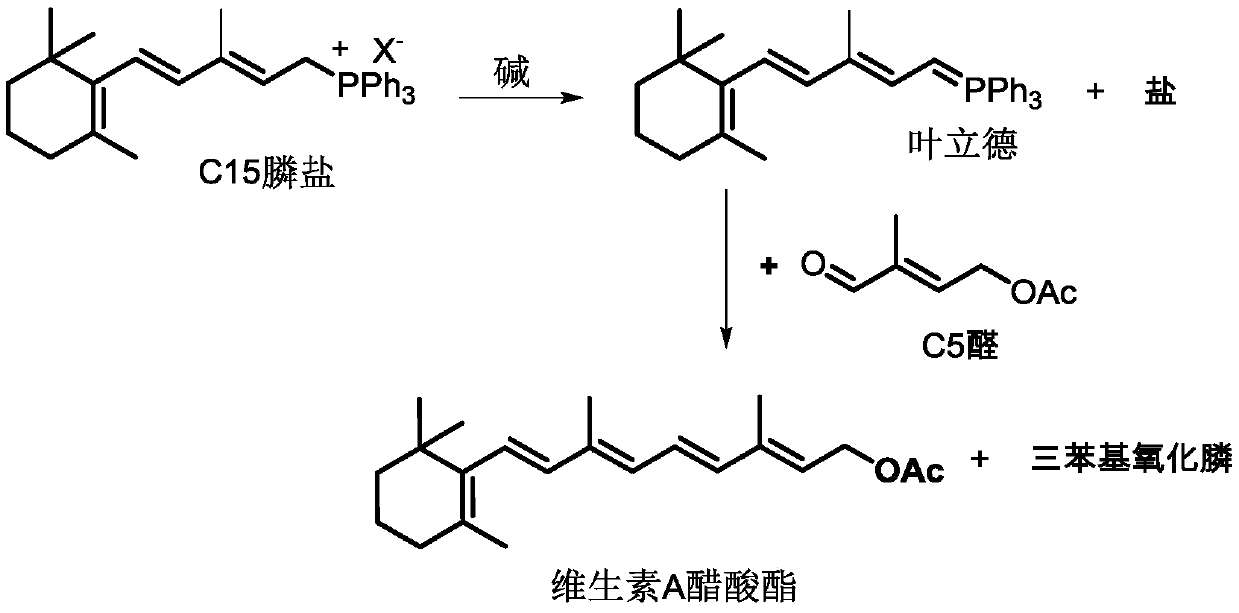
[0058] Preparation raw material F 1 , wherein the mass content of C15 phosphonium chloride is 30.37%, the mass content of C5 aldehyde is 8.88%, and the mass content of water is 60.75% (the molar ratio of C5 aldehyde to C15 phosphine chloride is 1.03). raw material F 1 Preheat to 40°C.
[0059] Prepare lye F 2 , wherein the sodium carbonate mass concentration is 15%, and the phase transfer aid cetyltrimethylammonium chloride mass concentration is 3% (the mol ratio of sodium carbonate and cetyltrimethylammonium chloride is 15), The rest is pure water. Lye F 2 Preheat to 40°C.
[0060] Petroleum ether was selected as the extractant E 0 . Extractant E 0 Preheat to 40°C.
[0061] Reactor such as figure 1 As shown, the raw material F 1 , lye F 2 and extractant E 0 The mass flow rates of 14.8kg / min, 7.0kg / min and 7.4kg / min are simultaneously passed into the first-stage reaction extraction stirring tank 1-1 and maintained at 40°C for the reaction extraction process. When ...
Embodiment 2
[0063] Preparation raw material F 1 , wherein the mass content of C15 phosphonium chloride is 15.77%, the mass content of C5 aldehyde is 5.37%, and the mass content of water is 78.86% (the molar ratio of C5 aldehyde to C15 phosphine chloride is 1.2). raw material F 1 Preheat to 55°C.
[0064] Prepare lye F 2 , wherein the mass concentration of sodium hydroxide is 35%, the mass concentration of phase transfer aid dodecyltrimethylammonium chloride is 6.4%, and all the other are pure water (sodium hydroxide and dodecyltrimethylammonium chloride The molar ratio is 36). Lye F 2 Preheat to 55°C.
[0065] Select n-heptane as extractant E 0 . Extractant E 0 Preheat to 55°C.
[0066] As shown in the figure, the raw material F 1 , lye F 2 and extractant E 0The mass flow rates of 38.0kg / min, 1.9kg / min and 9.4kg / min are simultaneously passed into the first-stage reaction extraction stirring tank 1-1 and maintained at 55°C for the reaction extraction process. When the liquid ho...
Embodiment 3
[0068] Preparation raw material F 1 , wherein the mass content of chloride C15 phosphine salt is 23.19%, the mass content of C5 aldehyde is 7.24% (the molar ratio of C5 aldehyde to chloride C15 phosphine salt is 1.1), and the water mass content is 69.57%. raw material F 1 Preheat to 50°C.
[0069] Prepare lye F 2 , wherein the mass concentration of potassium carbonate is 30%, the phase transfer aid cetyltrimethylammonium bromide mass concentration is 3.2%, and all the other are pure water (mole of potassium carbonate and cetyltrimethylammonium bromide than 25). Lye F 2 Preheat to 50°C.
[0070] Select n-hexane as extractant E 0 . Extractant E 0 Preheat to 50°C.
[0071] As shown in the attached figure, the raw material F 1 , lye F 2 and extractant E 0 The mass flow rates of 21.6kg / min, 5.5kg / min and 8.7kg / min are simultaneously passed into the first-stage reaction extraction stirring tank 1-1 and maintained at 50°C for the reaction extraction process. When the liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com