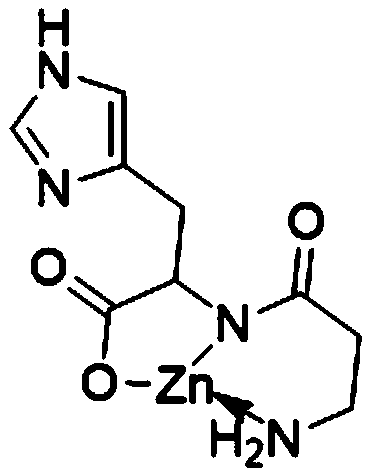
Preparation method of polaprezinc and polaprezinc preparation
A technology of polypurix zinc and reagents, which is applied in the preparation of polypurex zinc and the field of polypurex zinc preparations, can solve the problems of long filtration time and drying time, easy generation of impurities, serious environmental pollution, etc., and achieve prevention and treatment of digestion Sexual ulcer, no safety hazard, low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
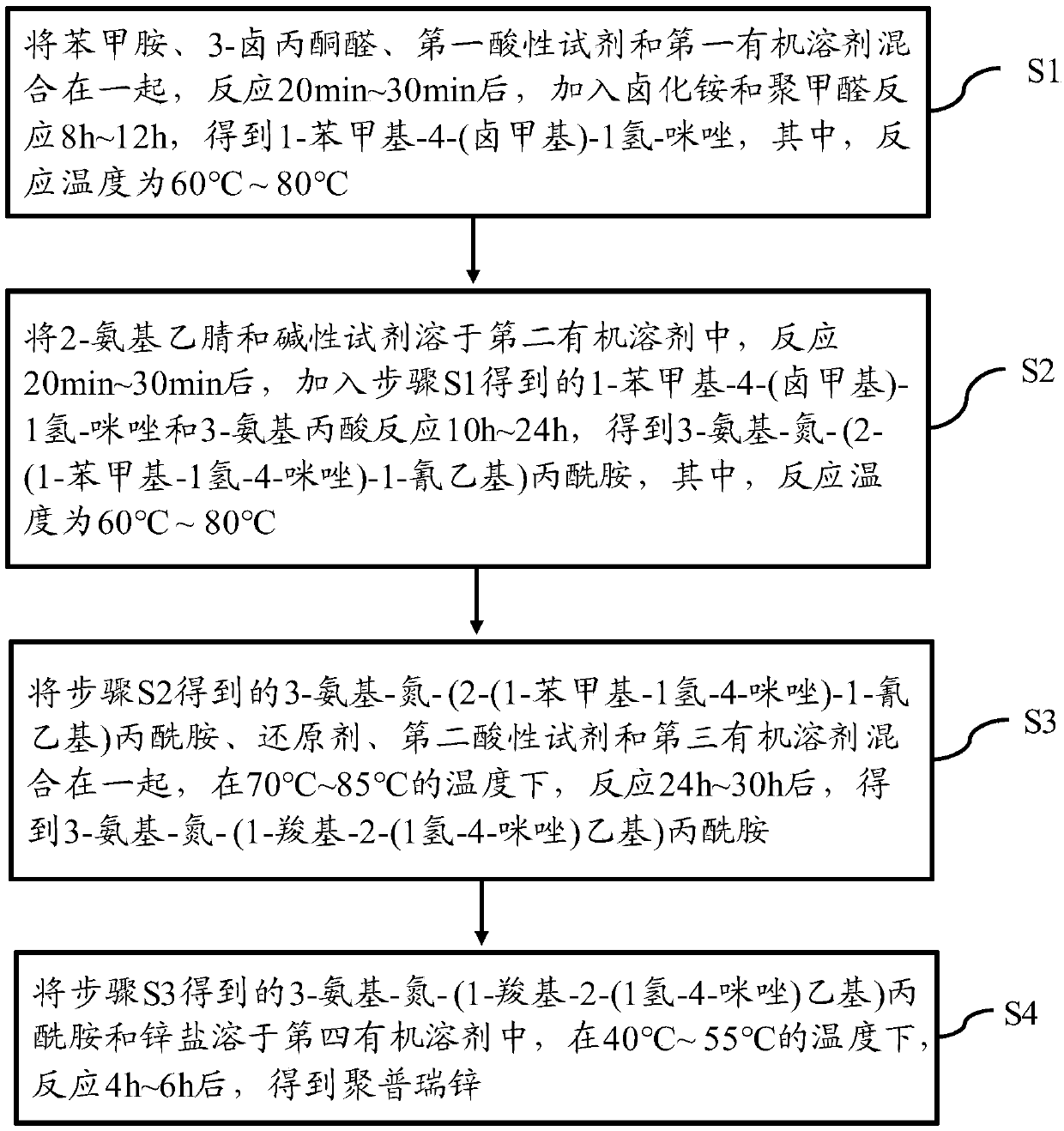
[0035] One embodiment of the present invention provides a kind of preparation method of polyprezinc, refer to figure 1 , figure 1 It is a flow chart of the preparation method of the polyprezinc of an embodiment of the present invention, and the preparation method of the polyprezinc comprises:
[0036]Step S1: Mix benzylamine, 3-haloacetone aldehyde, the first acidic reagent and the first organic solvent together, react for 20-30 minutes, add ammonium halide and polyoxymethylene to react for 8-12 hours, and obtain 1-benzyl -4-(halomethyl)-1 hydrogen-imidazole, wherein the reaction temperature is 60°C to 80°C;
[0037] Step S2: Dissolve 2-aminoacetonitrile and alkaline reagent in the second organic solvent, and after reacting for 20min to 30min, add 1-benzyl-4-(halomethyl)-1hydro-imidazole and 3-aminopropionic acid was reacted for 10h to 24h to obtain 3-amino-nitrogen-(2-(1-benzyl-1hydrogen-4-imidazole)-1-cyanoethyl)propionamide, wherein the reaction temperature was 60 ℃~80℃;...
Embodiment 1
[0052] The preparation method of the described polyprezinc of the present embodiment comprises the steps:
[0053] Step S1: Weigh 3.6g of benzylamine and 3.6g of 3-chloroacetoaldehyde, and weigh 0.3mL of hydrochloric acid and 130mL of methanol, mix them together and add them to a three-necked flask, heat up to 60°C, and react for 30 minutes, then transfer to the three-necked flask Add 1.8 g of ammonium chloride and 1.0 g of polyoxymethylene into the bottle, keep the reaction temperature at 60°C to 80°C, and continue the reaction for 8 hours. After the reaction was completed, the mixed solution was successively distilled under reduced pressure, washed with water, filtered and dried to obtain 5.7 g of 1-benzyl-4-(chloromethyl)-1 hydrogen-imidazole, and the calculated yield (in terms of benzyl Amine) was 83%.
[0054] Step S2: Weigh 1.5g of 2-aminoacetonitrile and 1.5g of potassium hydroxide, dissolve them in 130mL of tetrahydrofuran, add them to a three-necked flask, raise the ...
Embodiment 2
[0058] The preparation method of the described polyprezinc of the present embodiment comprises the steps:
[0059] Step S1: Weigh 3.6g of benzylamine and 5.1g of 3-bromoacetoglyoxal, and measure 0.2mL of phosphoric acid and 140mL of ethanol, mix them together and add them to a three-necked flask, raise the temperature to 70°C, and react for 30min. Add 3.3g of ammonium bromide and 1.0g of polyoxymethylene into the bottle, keep the reaction temperature at 70°C to 80°C, and continue the reaction for 10h. After the reaction was completed, the mixed solution was successively distilled under reduced pressure, washed with water, filtered and dried to obtain 6.3g of 1-benzyl-4-(bromomethyl)-1 hydrogen-imidazole, and the calculated yield (in terms of benzyl Amine) was 92%.
[0060] Step S2: Weigh 1.1g of 2-aminoacetonitrile and 1.1g of sodium methoxide, dissolve them in 140mL of tetrahydrofuran, add them to a three-necked flask, raise the temperature to 70°C, and react for 30 minutes, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com