Trifluoromethyl pyrimidine derivatives containing Schiff base structural unit, and preparation method and application thereof
A technology of trifluoromethylpyrimidine and structural units, which is applied in the field of medicinal chemistry and can solve problems such as drugs that cannot satisfy patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
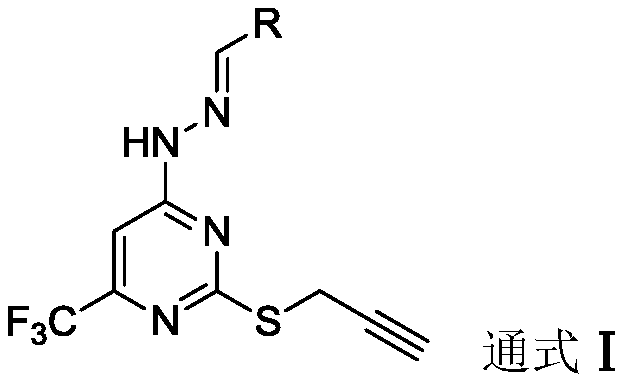
[0031] The preparation of embodiment 1 (E)-4-(2-phenylene dihydrazine)-2-(prop-2-yn-1-ylthio)-6-(trifluoromethyl)pyrimidine (5a)
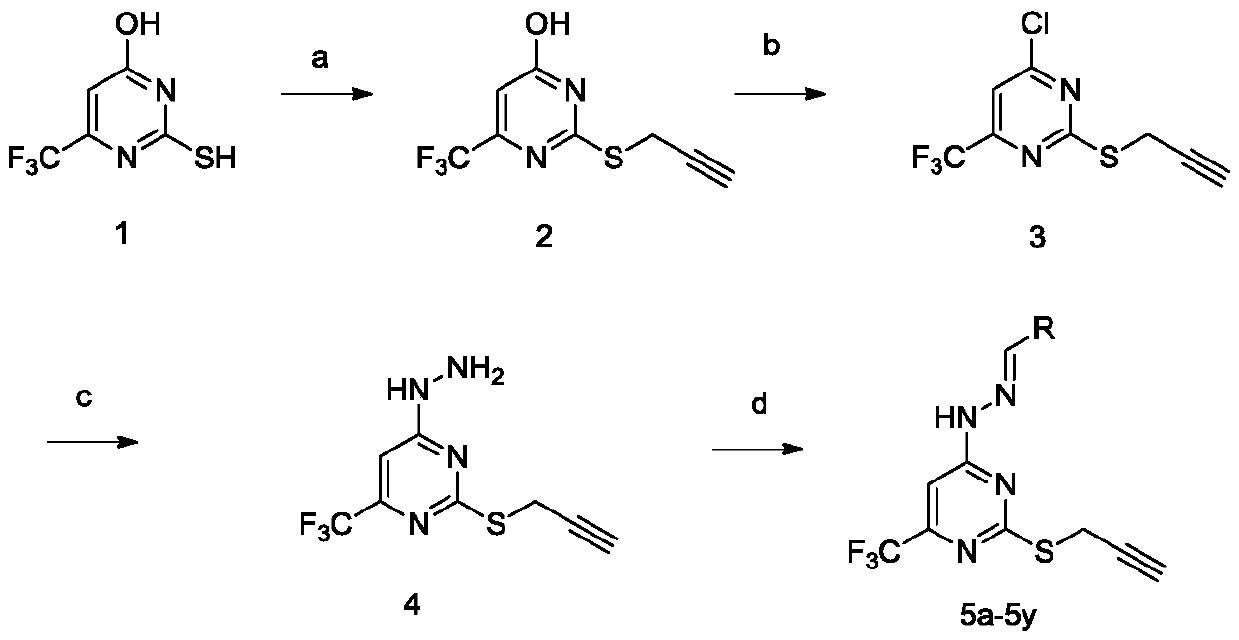
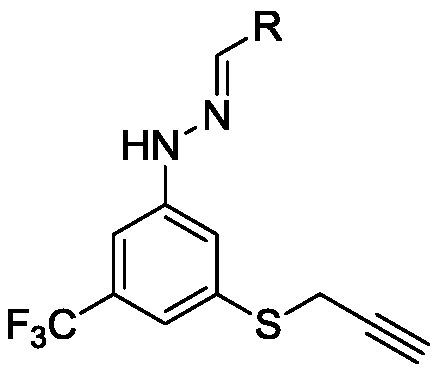
[0032] At room temperature, 2-mercapto-6-(trifluoromethyl)pyrimidin-4-ol (1.95g, 10mmol) was dissolved in potassium hydroxide (0.67g, 12.05mmol) in N,N-dimethylformyl (20ml) amine solution, slowly drop in propyne bromide (940ul, 12.05mmol), heat up to 80°C for a substitution reaction, TLC detects that the reaction is complete, cool the reaction solution to room temperature, add to the ice-water mixture and stir, there are a lot of pale A yellow solid was precipitated, filtered with suction, washed with water 3-4 times, and dried to obtain the compound 3-(prop-2-yn-1-ylthio)-5-(trifluoromethyl)phenol.
[0033] Pale yellow solid, yield 83.5%; 1 H NMR (400MHz, DMSO-d 6 )δ7.29(s,1H),5.19(d,J=2.3Hz,2H),4.82(d,J=2.3Hz,1H). 13 C NMR (101MHz, DMSO-d 6 )δ170.89, 168.65, 163.40, 159.39, 155.72, 155.37, 155.02, 154.66, 73.40, 55.37, 20.59, 19.17. HR-MS (E...
Embodiment 2
[0040] Example 2 (E)-4-(2-(3-fluorobenzylidene)hydrazino)-2-(prop-2-yn-1-ylthio)-6-(trifluoromethyl)pyrimidine Preparation of (5b)
[0041] At room temperature, (3-(prop-2-yn-1-ylsulfanyl)-5-(trifluoromethyl)phenyl)(0.15g, 0.609mmol)hydrazine was added to 6ml of absolute ethanol, and the temperature was raised to 80 DEG C, slowly add 3-fluorobenzaldehyde (60.97ul, 0.609mmol), TLC detects that the reaction is complete, the reaction solution is cooled to room temperature, and carries out column chromatography (V 石油醚 :V 乙酸乙酯 =6:1), the eluent was concentrated to obtain the product.
[0042] White solid, yield 74.0%; 1 H NMR (400MHz, DMSO-d 6 )δ 1 H NMR (400MHz, DMSO) δ12.26(s, 1H), 8.21(s, 1H), 7.67(d, J=9.8Hz, 1H), 7.59(d, J=7.8Hz, 1H), 7.49(dt ,J=13.8,6.9Hz,1H),7.34(s,1H),7.30–7.23(m,1H),3.99(d,J=2.6Hz,2H),3.18(t,J=2.6Hz,1H) . 13 C NMR (101MHz, DMSO-d 6 )δ169.92, 163.66, 162.15, 161.23, 144.41, 136.43, 130.88, 130.79, 123.59, 116.89, 116.68, 113.10, 112.88, 96.17, 79.8...
Embodiment 3
[0043] Example 3 (E)-4-(2-(4-fluorobenzylidene)hydrazino)-2-(prop-2-yn-1-ylthio)-6-(trifluoromethyl)pyrimidine Preparation of (5c)
[0044] Replace 3-fluorobenzaldehyde with 4-fluorobenzaldehyde, and the preparation method is the same as in Example 2.
[0045] White solid powder, yield 62.4%; 1 H NMR (400MHz, DMSO-d 6 )δ12.19(s,1H),8.22(s,1H),8.05–8.00(m,4H),7.33(s,1H),4.00(d,J=2.5Hz,2H),3.19(t,J =2.6Hz,1H). 13 C NMR (101MHz, DMSO-d 6 )δ170.09,161.97,152.63,147.84,141.66,127.28,124.40,122.01,119.16,117.08,114.31,95.70,79.91,73.40,61.18,55.75,18.57. 15 h 11 f 4 N 4 S[M+H] + :355.0641,found:355.0642.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com