Method for preparing oxazole compound
A technology of compounds and oxazoles, which is applied in the field of organic synthesis, can solve the problems of large by-products and low yield of target products, and achieve the effects of short reaction time, mild reaction conditions and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] According to the method for preparing oxazole compounds provided by the present invention, it has the following reaction formula:
[0044]
[0045] The inventors of the present invention have proved according to experimental results that when using phosgene or diphosgene or triphosgene to prepare oxazole compounds, adding a phosphorus-containing or boron-containing compound with a specific structure can promote the efficient progress of the main reaction, while the side effects The reaction is inhibited. The inventors have found through experimental research that according to the method for preparing oxazole compounds provided by the present invention, the target product oxazole compounds represented by formula (V) can be obtained in high yield, and the formation of by-products can be greatly suppressed at the same time.
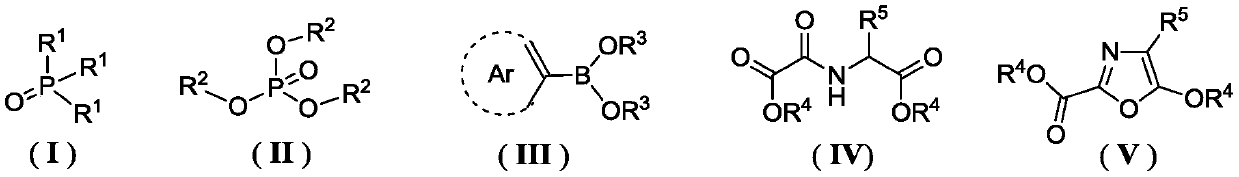
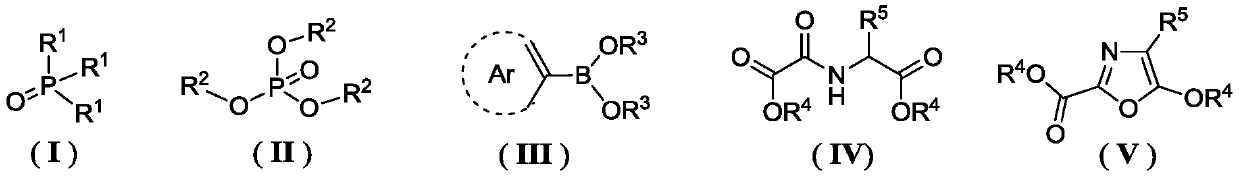
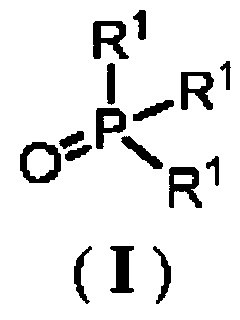
[0046] According to the method for the preparation of oxazole compounds provided by the present invention, the trihydrocarbyl phosphine oxide repr...
Embodiment 1
[0062] (using phenylboronic acid as auxiliary agent):
[0063] In the reaction flask, add 24g N-ethoxyoxalylalanine ethyl ester (0.11mol), 0.14g phenylboronic acid (1.1mmol) and 42.4g triethylamine (0.42mol), after dissolving with 45mL chloroform, Triphosgene solution (13.1g, 0.044mol, dissolved in 50mL chloroform) was added dropwise at 0-5°C with stirring, and the dropwise addition was completed in about 1 hour, then heated to 50°C for 1 hour, followed by gas chromatography and found that The raw material N-ethoxyoxalylalanine ethyl ester was completely consumed. After reaction finishes, detect by gas chromatography, confirm that gained product is 4-methyl-5-ethoxy oxazole, and its internal standard molar yield is 96% (yield is based on N-ethoxy oxalylalanine ethyl Esters, detected by internal standard method, n-octadecane is internal standard), while only generating 3% (molar yield) of N,N-diethylformyl chloride (yield is based on 3 times of triphosgene, detected by externa...
Embodiment 2-1
[0068] (Using triethyl phosphate as auxiliary agent):
[0069] In the reaction flask, add 30g N-butoxy oxalylalanine n-butyl ester (0.11mol), 0.9g triethyl phosphite (5.5mmol) and 42.4g triethylamine (0.42mol), and use 45mL tris After the methyl chloride is dissolved, triphosgene solution (13.1g, 0.044mol, dissolved in 50mL chloroform) is added dropwise at 0-5°C with stirring, and the dropwise addition is completed in about 1 hour, and then heated to 50°C for 1 hour to react. Gas chromatography followed the reaction and found that the raw material N-butyloxyoxalylalanine n-butyl ester was completely consumed. After reaction finishes, detect by gas chromatography, confirm that gained product is 4-methyl-5-n-butoxy oxazole, and its internal standard molar yield is 95% (yield is based on N-butoxy oxalyl alanamide N-butyl acid, internal standard method detection, n-octadecane is the internal standard), and only generate 4% (molar yield) of N,N-diethylformyl chloride (yield is bas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com