Methods and compositions for treating respiratory disease
A composition and virus technology, applied in the field of treatment of respiratory diseases and compositions, can solve the problems of medical resource burden, harmful effects on patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
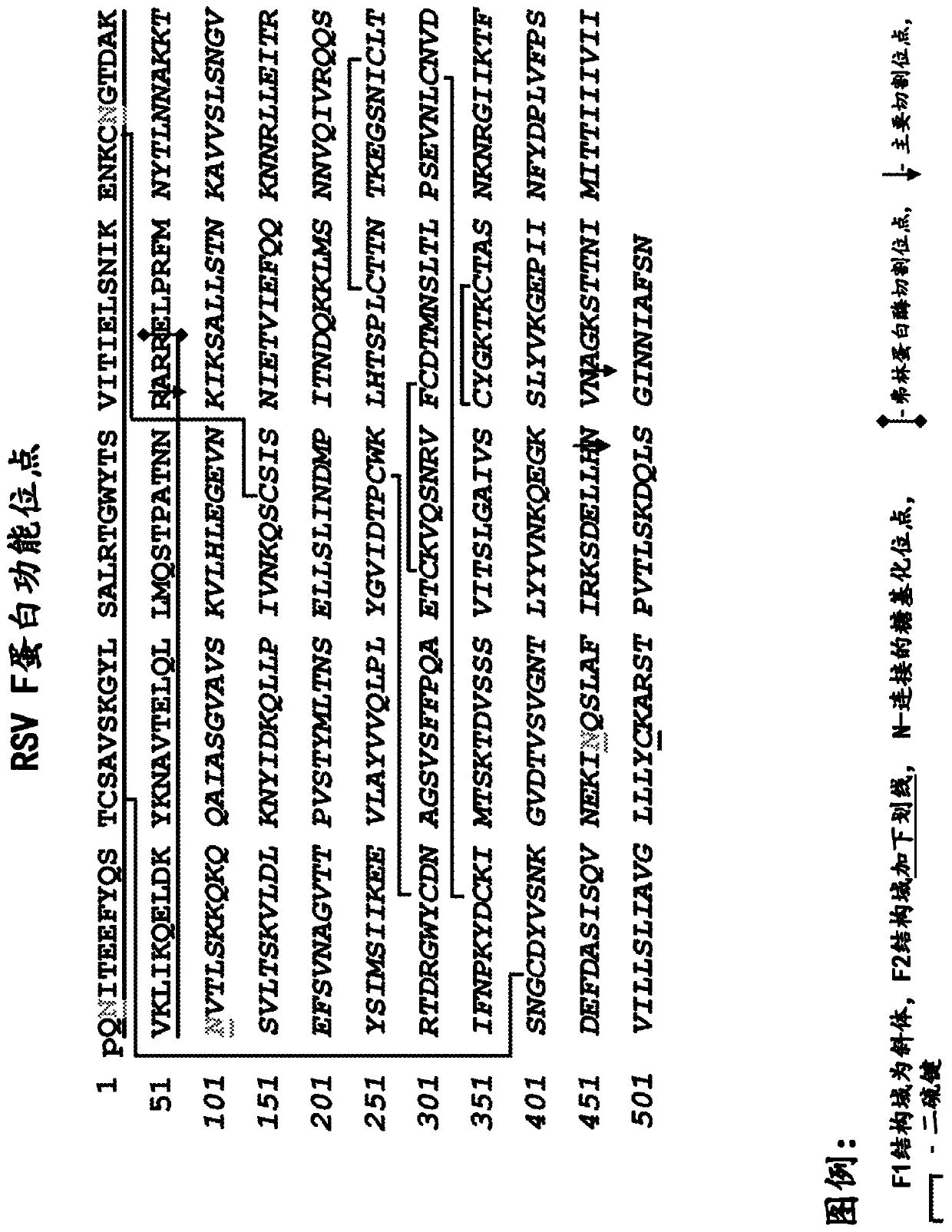
[0135] Expression and purification of RSV F protein
[0136] The RSV F protein having SEQ ID NO: 8 was expressed in a baculovirus expression system, and recombinant plaques expressing the RSV F protein were selected and confirmed. The recombinant virus was then amplified by infecting Sf9 insect cells. Insect cell cultures were infected with baculovirus at approximately 3 MOI (multiplicity of infection = virus ffu or pfu / cell). Cultures and supernatants were harvested 48-72 hours after infection. Approximately 30 mL of crude cell harvest was clarified by centrifugation at approximately 800 xg for 15 minutes. The resulting crude cell harvest containing RSV F protein was purified as described below.
[0137] Using Nonionic Surfactants in Membrane Protein Extraction Protocols NP-9 (nonylphenol ethoxylate). NP-9 was a crude extract which was further purified by anion exchange chromatography, lentil lectin affinity / HIC and cation exchange chromatography. Washed cells were lys...
Embodiment 2
[0142] Preparation of vaccine composition
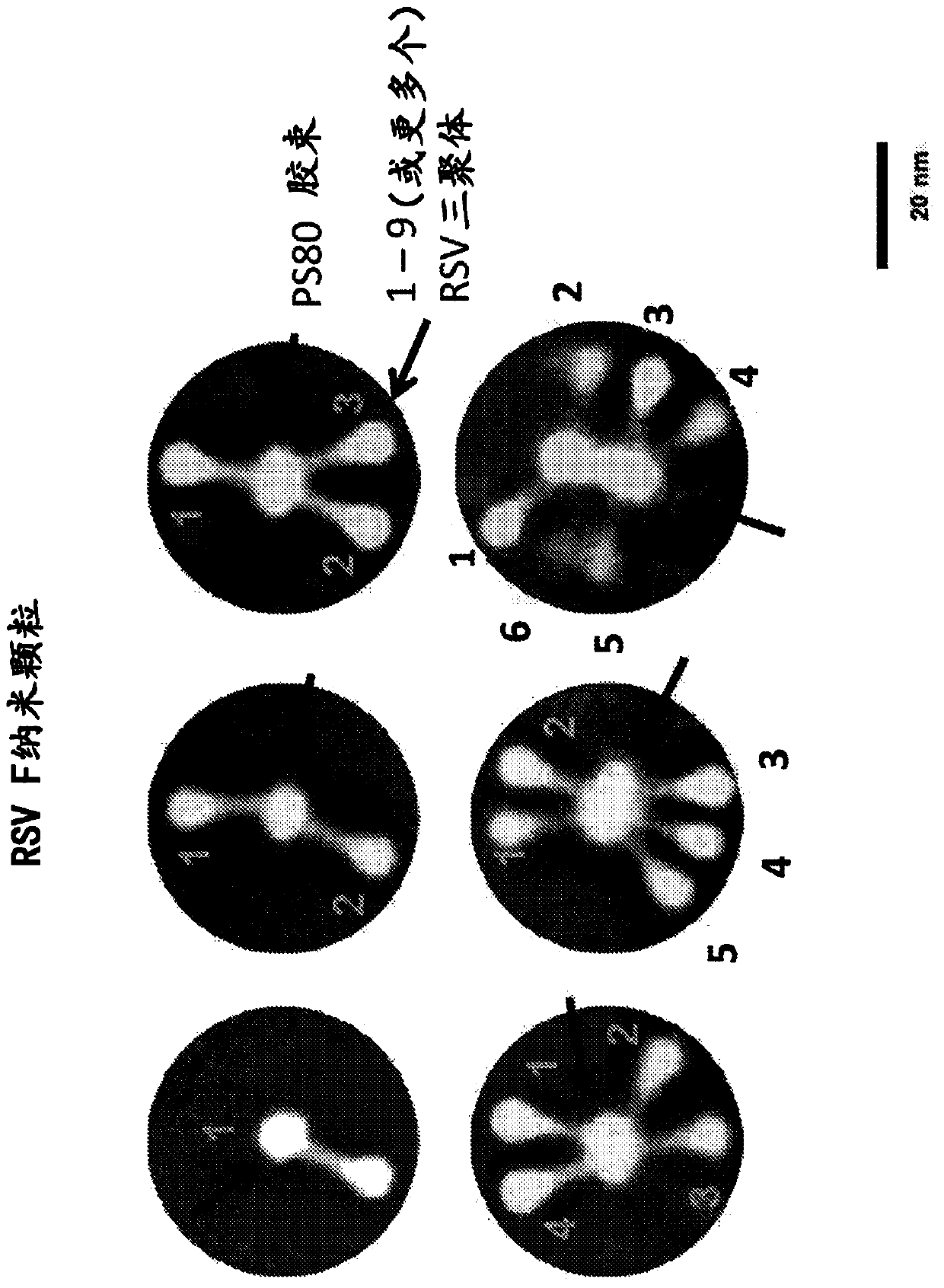
[0143]To provide the nanoparticles for administration of the vaccine product, the drug substance is diluted into the drug product with a PS80:RSV protein molar ratio of about 50. Prior to administration, the drug substance is thawed, diluted and filled into glass vials or prefilled syringes for storage at 2°C-8°C. Nanoparticles are combined with alum adjuvant. Alum adjuvant was added and mixed to ensure that about 95% of the nanoparticles were bound to alum, meaning about 0.4 mg per 120 μg dose of RSV F nanoparticles in a volume of 0.5 mL.
Embodiment 3
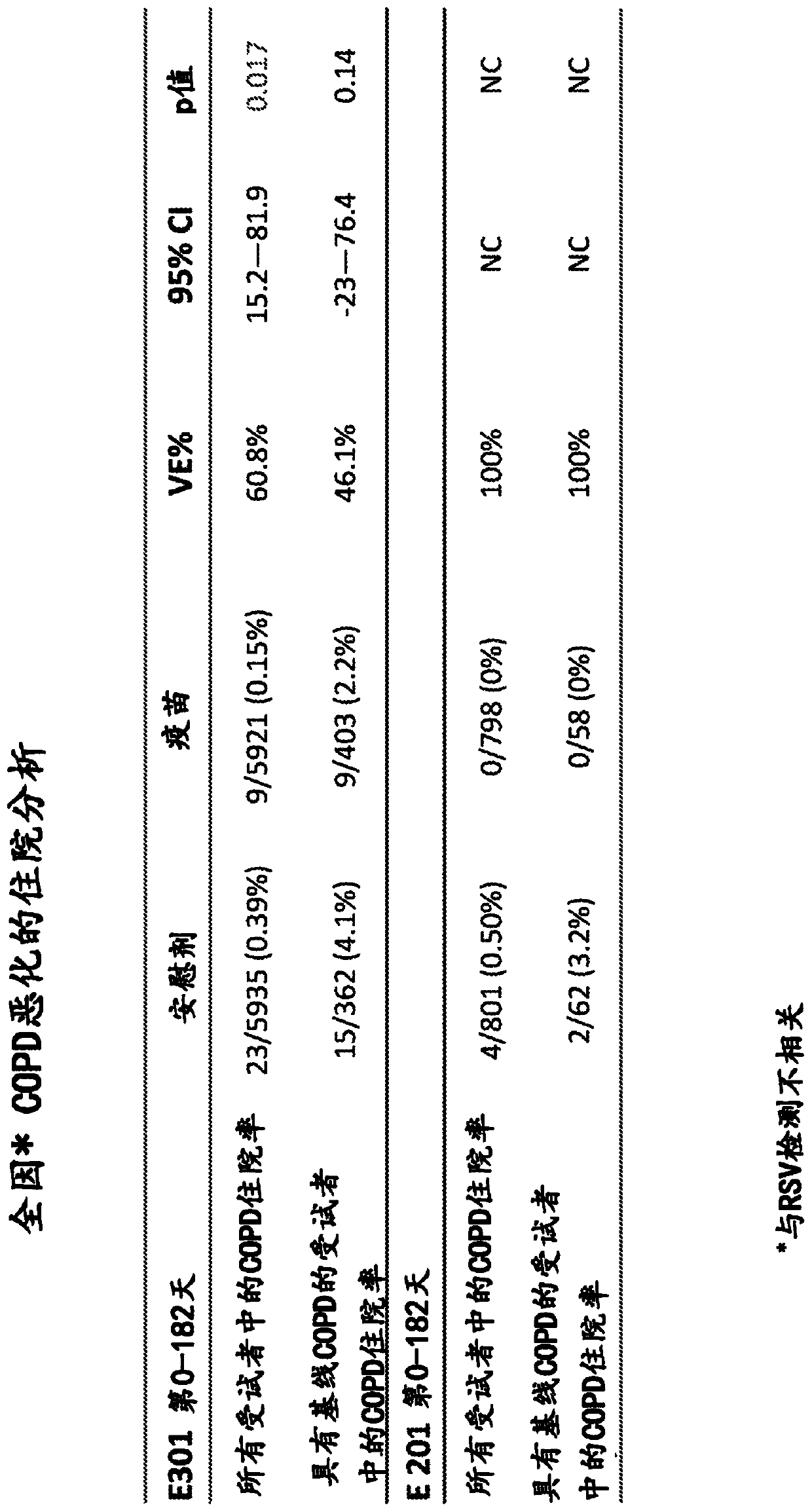
[0145] RSV F in nanoparticles reduces COPD exacerbation hospitalizations
[0146] In a randomized placebo-controlled study of 11,856 subjects aged over 60 years, a vaccine containing 135 μg of RSV F nanoparticles in the absence of alum reduced all-cause COPD exacerbation hospitalizations (in image 3 and Figure 4 referred to as "E-301"). Subjects were followed for one year to determine the safety, immunogenicity and efficacy of the vaccine. Surprisingly, administration of the RSV F nanoparticle vaccine reduced both the general population of the study (p=0.017) and the subpopulation of the study (p=0.14) in subjects previously identified as having baseline COPD. Hospitalization due to exacerbation.
[0147] In a smaller comparative study consisting of 1600 subjects aged over 60 years, the group given the RSV F nanoparticle vaccine did not experience COPD exacerbation hospitalization ( image 3 referred to as "E-201"). Data show that in the E-201 study, 0 of 798 subjects w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com