Specific TCR pointing to EGFR L858R gene mutation and application of specific TCR
A specific, KITDFGRAK-HLA-A1101 technology, applied in the field of genetic engineering and tumor immunotherapy, can solve the problems of clinical treatment effect to be verified, lack of clinical trial data, etc., and achieve good therapeutic effect and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
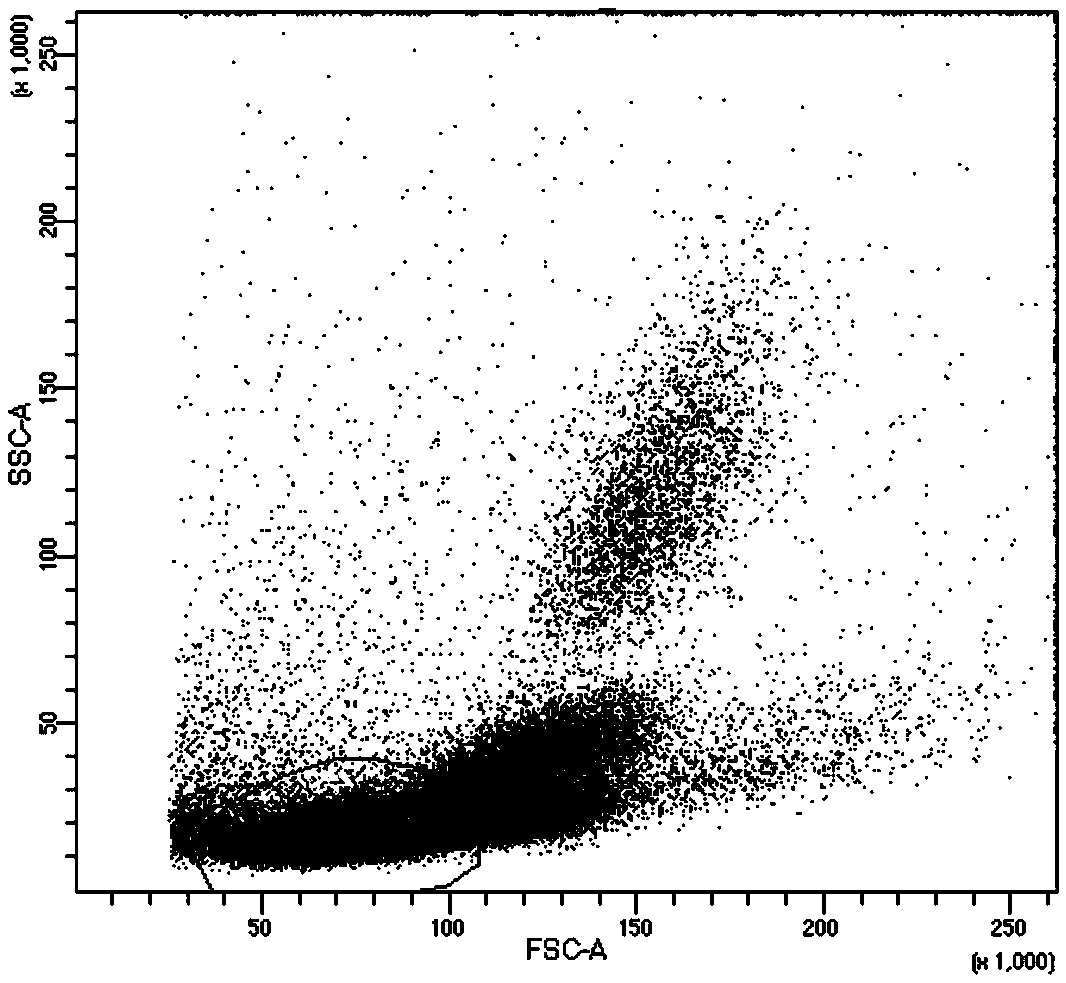
[0041]Example 1 Flow cytometry analysis of the TCR expression of the constructed TCR-T
[0042] According to the TCR gene sequence obtained by sequencing, the TCR α chain and β chain are fully synthesized. Digest the pMX-IRES-GFP plasmid with Not1 and EcoR1 restriction endonucleases, recover the empty vector gene fragment from the gel; dephosphorylate the virus plasmid with alkaline phosphatase (CIAP), and the reaction system is 50ul: plasmid 20ul, 10×Buffer 5ul , CIAP 2ul, sterile water 23ul, incubated at 37°C for 30min, and purified plasmid DNA by ethanol precipitation. The TCR full-length gene was ligated into pMX-IRES-GFP empty vector plasmid, and ligated overnight at 16°C. Transform the recombinant plasmid into XL-10 competent cells, evenly spread it on the LB solid medium plate containing ampicillin, culture at 37°C for 12 hours, pick a single colony into the LB liquid medium containing ampicillin, and incubate at 37°C , 220rpm / min shaking culture for 14-16h, extract t...
Embodiment 2
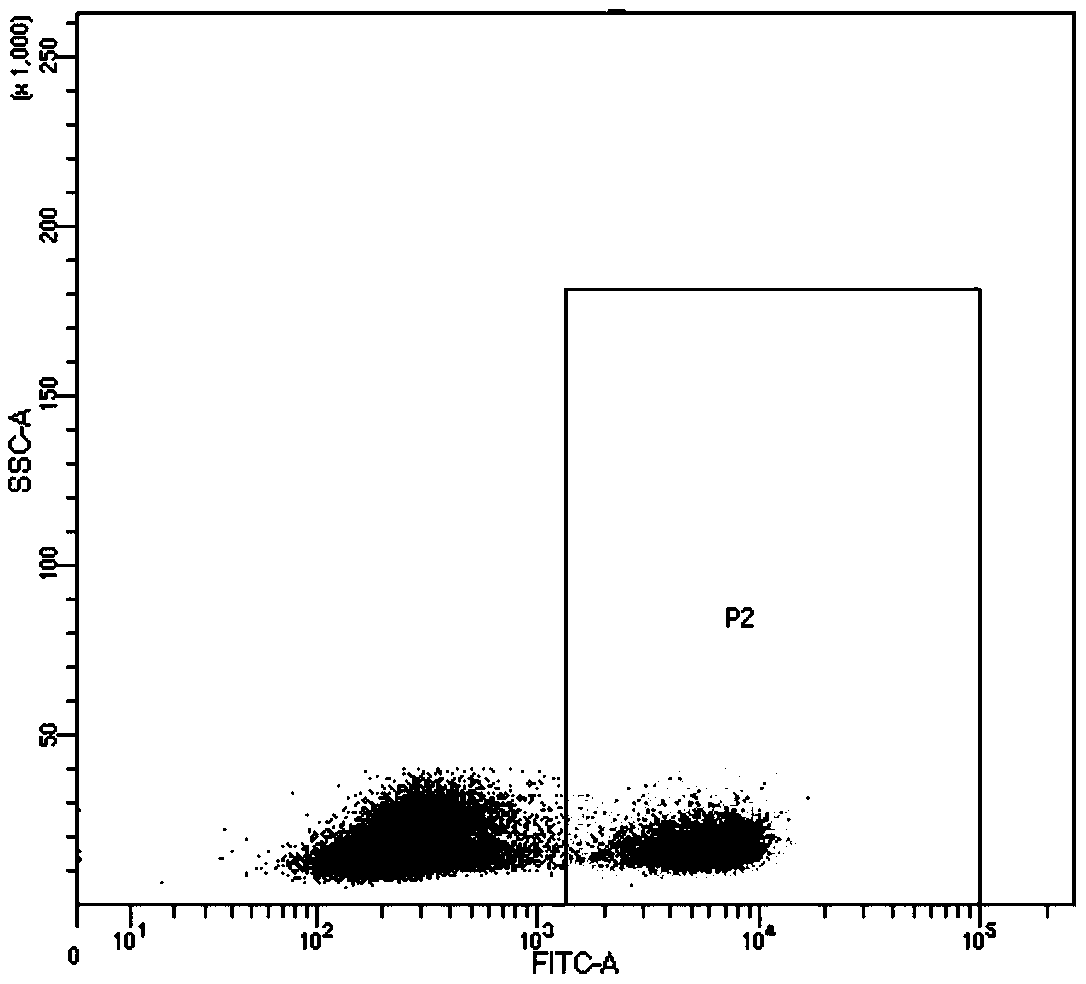
[0045] Example 2 Flow Cytometry Analysis of the Ability of Specific TCR-T to Recognize Specific Antigen Peptides
[0046] Take peripheral blood from healthy volunteers, and separate PBMCs by density gradient centrifugation: Peripheral blood is diluted with PBS at a ratio of 1:1, and the diluted blood is carefully added to the lymphocyte separation medium at a ratio of 1:1 to form an obvious layer. Centrifuge horizontally at 1600rpm / min for 25min. Aspirate the mononuclear cell layer carefully with a pipette, wash the cells twice with serum-free 1640 medium, resuspend the cells and count them. Human peripheral blood mononuclear cells (PBMCs) were activated for 2 days with anti-CD3 antibody (20 ng / mL) and interleukin 2 (IL-2; 300 IU / ml). The day before the infection, the PBMC cells in the logarithmic growth phase were pipetted into a single cell suspension and counted 5×10 5 2 cells were seeded in a 6-well plate coated with fibronectin. On the day of infection, 200ul concentrat...
Embodiment 3
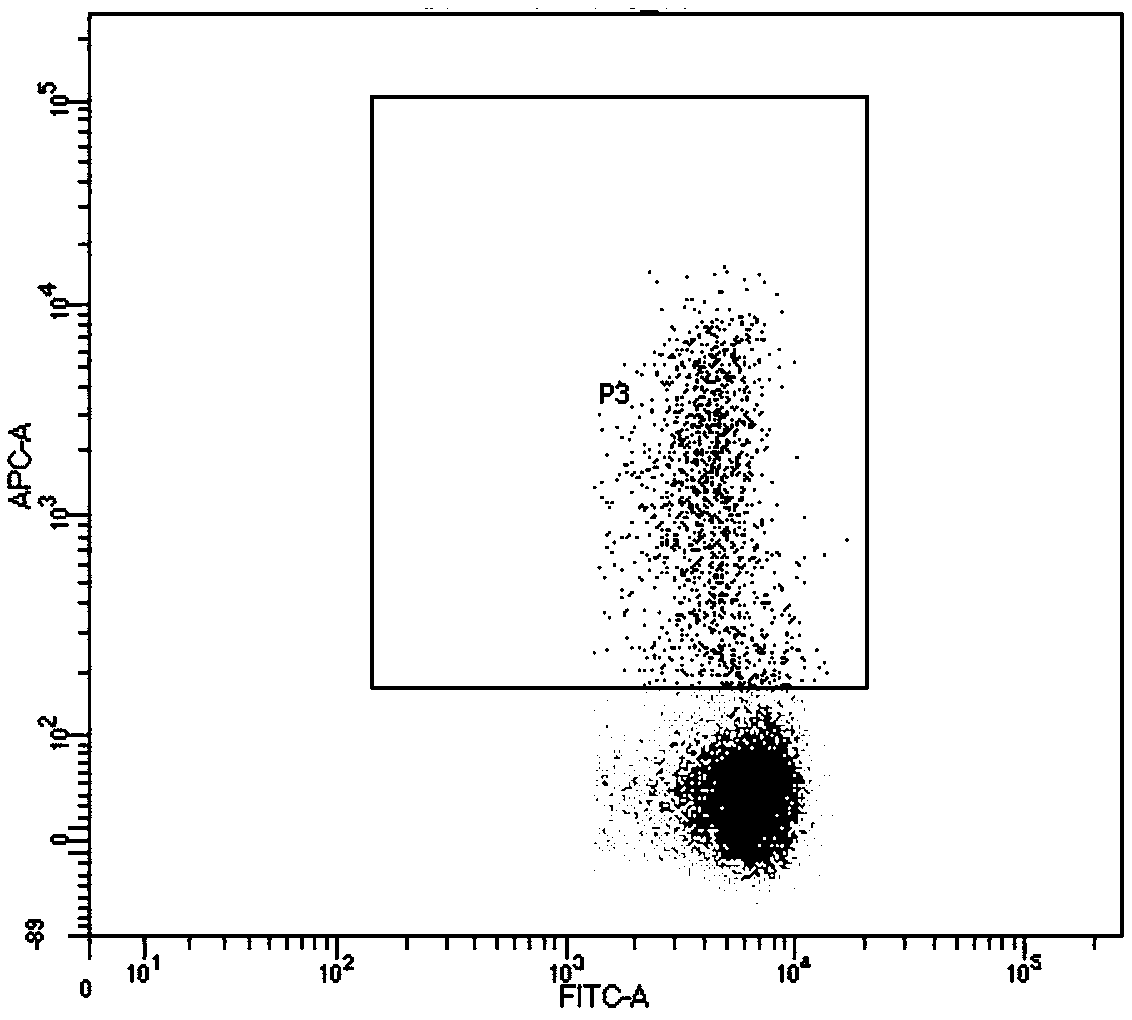
[0047] Example 3 Flow Cytometry Analysis of the Killing Effect of Specific TCR-T Cells on Target Cells
[0048] The T2 cell line of HLA-A1101 is constructed. The T2 cell line lacks transporters related to antigen processing (TAP), so it can effectively load foreign peptides, and as antigen presenting cells, present the loaded antigen peptides to T cells for recognition. The artificially synthesized antigen peptide KITDFGRAK was mixed with T2 cells at 37°C, 5% CO 2 Incubate for 24 h under the conditions (the concentration of polypeptide is 50 μg / ml, the concentration of T2 cells is 1×10 6 cells / ml), washed to remove unbound antigenic peptides, and then the cells were collected, which were T2 cells loaded with antigenic peptide KITDFGRAK.
[0049] Specific TCR-T cells and T2 cells loaded with antigen peptide KITDFGRAK were incubated at 37°C, 5% CO 2 Incubate for 24 h under the condition (the cell concentration is 1×10 6 pieces / ml). Control cells were T2 cells not loaded with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com