2,5-dimethylfuran and method for preparing 2,5-dimethylfuran by hydrogenation of 5-hydroxymethylfurfural
A technology of dimethylfuran and hydroxymethylfurfural, applied in directions such as organic chemistry, can solve problems such as increasing operating costs, achieve the effects of saving costs, avoiding the use of precious metal catalysts, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of catalyst includes but not limited to following method:
[0026] 21.10g Mg(NO 3 ) 2 ·6H 2 O, 4.71g Zr(NO 3 ) 4 ·5H 2 O and 9.94g Cu(NO 3 ) 2 ·3H 2 O was added to a beaker containing 200 mL of deionized water to make Mg(NO 3 ) 2 ·6H 2 O: Zr(NO 3 ) 4 ·5H 2 O: Cu(NO 3 ) 2 ·3H 2 O=8:1:4 (the ratio of the amount of substances). Seal the mouth of the beaker with a parafilm to avoid liquid splashing, and stir at a speed of 500r / min until all the solids are dissolved, and this operation is carried out at room temperature.
[0027] Configure 1mol / L Na 2 CO 3 Solution 200mL, ready to use.
[0028] Na 2 CO 3 The solution was added dropwise to the prepared Mg(NO 3 ) 2 , Zr(NO 3 ) 4 and Cu(NO 3 ) 2 in the mixed solution, and always control the pH=10 of the solution.
[0029] After the pH was stabilized, the stirring was continued for 2 hours, then the stirring was stopped, and left to age for 24 hours.
[0030] The aged catalyst...
Embodiment 1
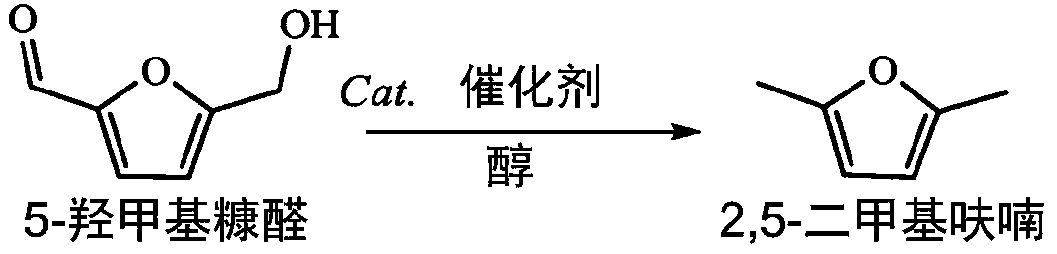
[0037] The method for preparing 2,5-dimethylfuran by hydrogenation of 5-hydroxymethylfurfural in this embodiment comprises the following steps:
[0038] 0.2mmol 5-hydroxymethylfurfural, 3mL isopropanol, 20mg CuO / MgO-ZrO 2 Put it into a 10mL stainless steel reactor, and use nitrogen to purge the reactor for 10s, and finally seal the reactor, react at 250°C for 3h, take out the solid-liquid mixture after the reaction and separate to obtain 2,5-dimethylfuran solution.
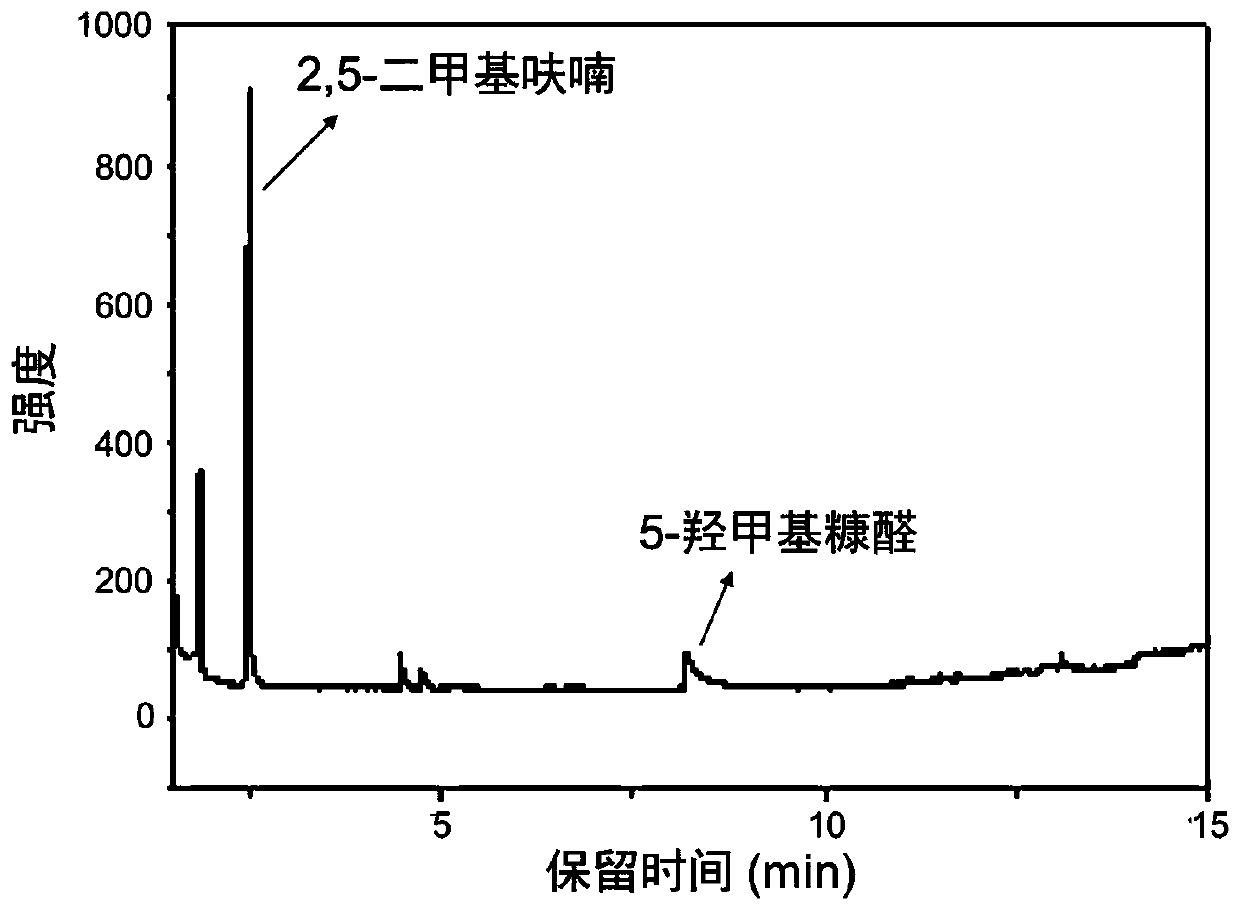
[0039] The reacted product was analyzed by gas chromatography (see figure 1), the results of gas chromatography showed that the yield of 2,5-dimethylfuran was 54.8%. In industrial applications, a suitable pressure-resistant reactor can be used as required, and the reaction temperature can be controlled at 250°C for 3 hours. Through this reaction, 5-hydroxymethylfurfural can be synthesized into 2,5-dimethylfuran, the operation is simple and the reaction selectivity is good.
Embodiment 2
[0041] The method for preparing 2,5-dimethylfuran by hydrogenation of 5-hydroxymethylfurfural in this embodiment comprises the following steps:
[0042] 0.2mmol 5-hydroxymethylfurfural, 3mL methanol, 20mg CuO / MgO-ZrO 2 Put it into a 10mL stainless steel reactor, and use nitrogen to purge the reactor for 10s, and finally seal the reactor, react at 250°C for 3h, take out the solid-liquid mixture after the reaction and separate to obtain 2,5-dimethylfuran solution.
[0043] The reacted product was analyzed by gas chromatography, and the result of gas chromatography showed that the yield of 2,5-dimethylfuran was 34.8%. In industrial applications, a suitable pressure-resistant reactor can be used as required, and the reaction temperature can be controlled at 250°C for 3 hours. Through this reaction, 5-hydroxymethylfurfural can be synthesized into 2,5-dimethylfuran, the operation is simple and the reaction selectivity is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com