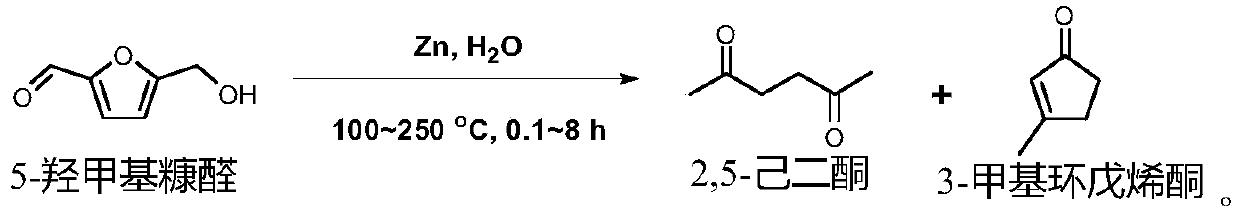Utilize 5-hydroxymethylfurfural to prepare the method for 2,5-hexanedione and 3-methylcyclopentenone
A technology of methylcyclopentenone and hydroxymethylfurfural, which is applied in the preparation of heterocyclic compounds, organic chemistry and other directions, can solve problems such as low yield, and achieve the effects of less reaction by-products, good selectivity and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This example relates to a method for synthesizing 2,5-hexanedione and 3-methylcyclopentenone by hydrothermally converting 5-hydroxymethylfurfural into a metal element (zinc powder) as a raw material, comprising the following steps:
[0030] 5-Hydroxymethylfurfural (0.2mmol), zinc powder (25mmol) and water (7.5mL) were successively loaded into a Teflon-lined reactor, and the reactor was filled with nitrogen to remove the air and then sealed, and the reactor was placed Put it into an oven so that the reaction temperature is 250°C, and the reaction time is 140 minutes. After the reaction, the solid-liquid mixture is taken out and separated to obtain 2,5-hexanedione and 3-methylcyclopentenone.
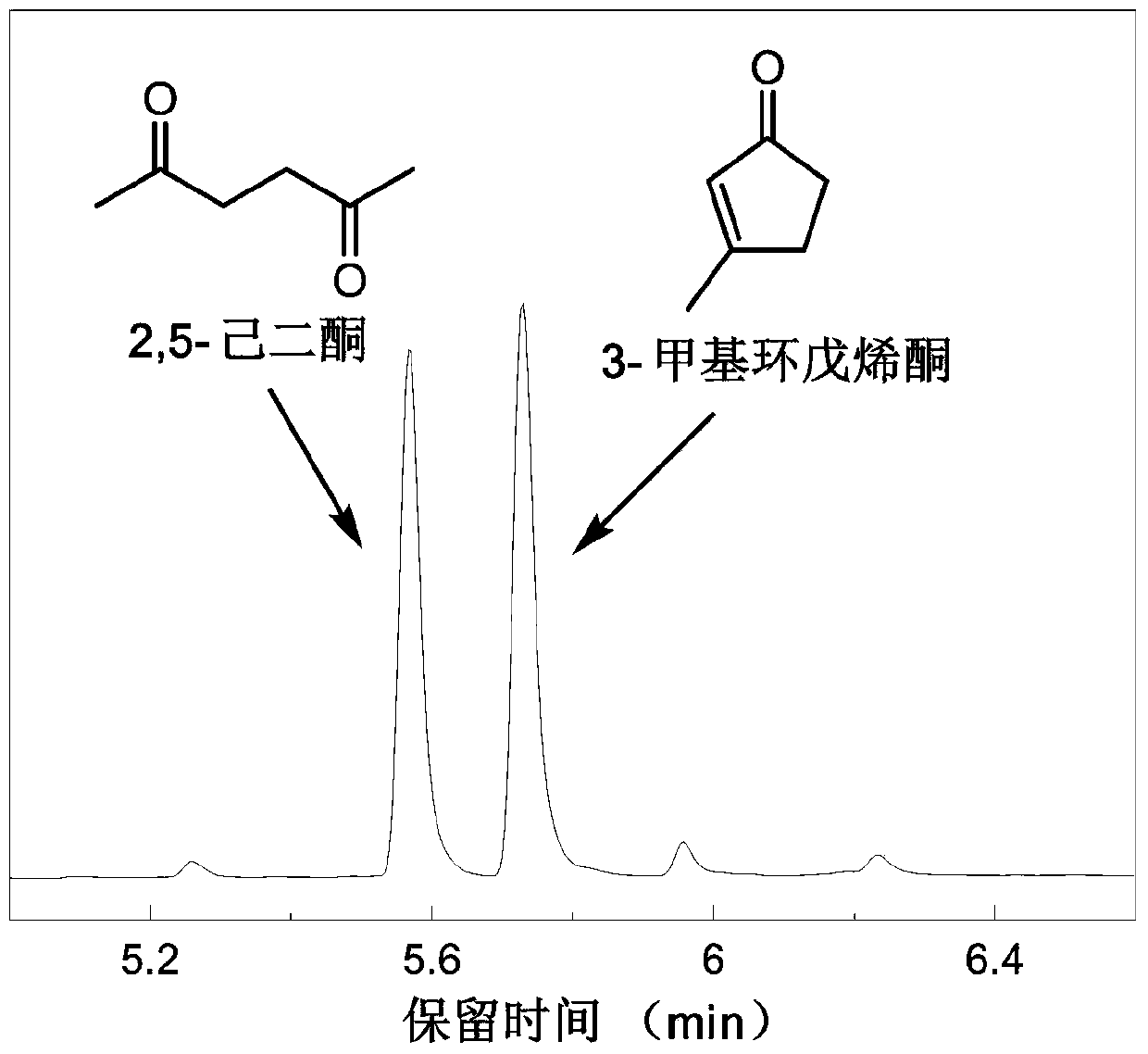
[0031] After the reaction, the product was analyzed by GC / MS (see figure 1 ), GC / MS analysis shows that 2,5-hexanedione and 3-methylcyclopentenone are the main products, and the yield of 2,5-hexanedione can reach 27.3%, and 3-methylcyclopentenene The ketone yield can reach up to 25...
Embodiment 2
[0034] This example relates to the hydrothermal conversion of a metal element (zinc powder) to 5-hydroxymethylfurfural as a raw material to synthesize 2,5-hexanedione and 3-methylcyclopentenone. The reaction equation is as follows:
[0035] 5-Hydroxymethylfurfural (0.2mmol), zinc powder (25mmol) and water (7.5mL) were successively loaded into a Teflon-lined reactor, and the reactor was filled with nitrogen to remove the air and then sealed, and the reactor was placed Put it into an oven so that the reaction temperature is 250°C, and the reaction time is 8 hours. After the reaction, the solid-liquid mixture is taken out and separated to obtain 2,5-hexanedione and 3-methylcyclopentenone.
[0036] The product after the reaction was analyzed by GC / MS. GC / MS analysis showed that 2,5-hexanedione and 3-methylcyclopentenone were the main products, and the yield of 2,5-hexanedione was up to 20.4 %, the highest yield of 3-methylcyclopentenone can reach 30.5%. In industrial applications...
Embodiment 3
[0039] This example relates to the hydrothermal conversion of a metal element (zinc powder) to 5-hydroxymethylfurfural as a raw material to synthesize 2,5-hexanedione and 3-methylcyclopentenone. The reaction equation is as follows:
[0040] 5-Hydroxymethylfurfural (0.2mmol), zinc powder (15mmol) and water (7.5mL) were successively loaded into a Teflon-lined reactor, and the reactor was filled with nitrogen to remove the air and then sealed, and the reactor was placed Put it into an oven so that the reaction temperature is 250°C, and the reaction time is 1 hour. After the reaction, the solid-liquid mixture is taken out and separated to obtain 2,5-hexanedione and 3-methylcyclopentenone.
[0041] The post-reaction product was analyzed by GC / MS. GC / MS analysis showed that 2,5-hexanedione and 3-methylcyclopentenone were the main products, and the yield of 2,5-hexanedione was as high as 19.4 %, the highest yield of 3-methylcyclopentenone can reach 15.6%. In industrial applications,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com