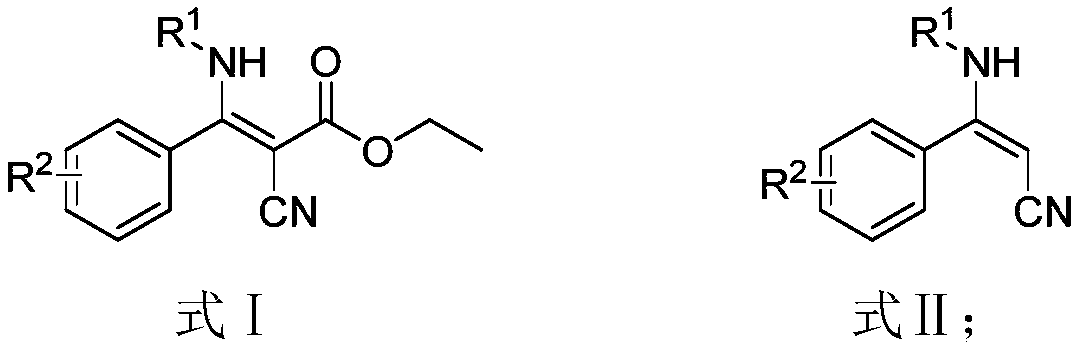
Preparation method of beta-amino acrylonitrile compounds
An aminoacrylonitrile and compound technology, which is applied in the field of preparation of beta-aminoacrylonitrile compounds, can solve problems such as the inability to well solve the problem of product geometry selectivity, and achieves simplified and smooth method, good yield, and three wastes. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of (E) 3-phenyl-3-(phenylamino)-acrylonitrile (l)
[0037] Add β-aminocyanoacrylate (8mmol, 1eq), lithium chloride (16mmol, 2eq), dimethyl sulfoxide (40mL), water (10mL) in sequence into a 100mL three-neck flask as shown in formula 1-1 , stir to dissolve, and heat to 160°C for 12 hours until the reaction of the raw materials is complete. After cooling to room temperature (25°C), 100 mL of ethyl acetate was added, the organic phase was washed with (2×50 mL) water and 50 mL of saturated saline solution, and the crude product was washed with petroleum ether: ethyl acetate = 4:1 after precipitation under reduced pressure. Recrystallization gave 1.23 g of white solid (E) 3-phenyl-3-(phenylamino)-acrylonitrile (1 in Table 1, formula 1), with a yield of 70.0%.
[0038]
[0039] The product has undergone a single crystal structure, 1 H NMR, 13 C NMR and MS confirmed:
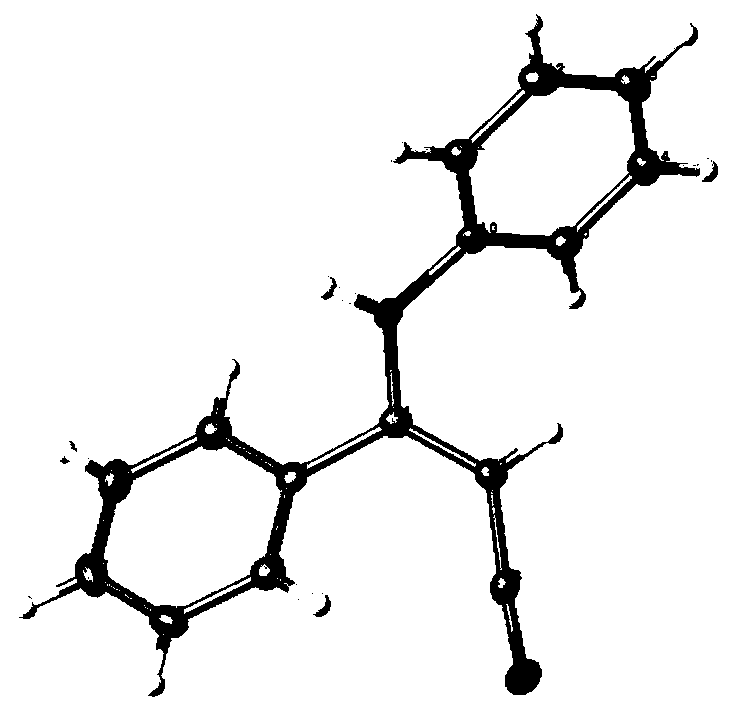
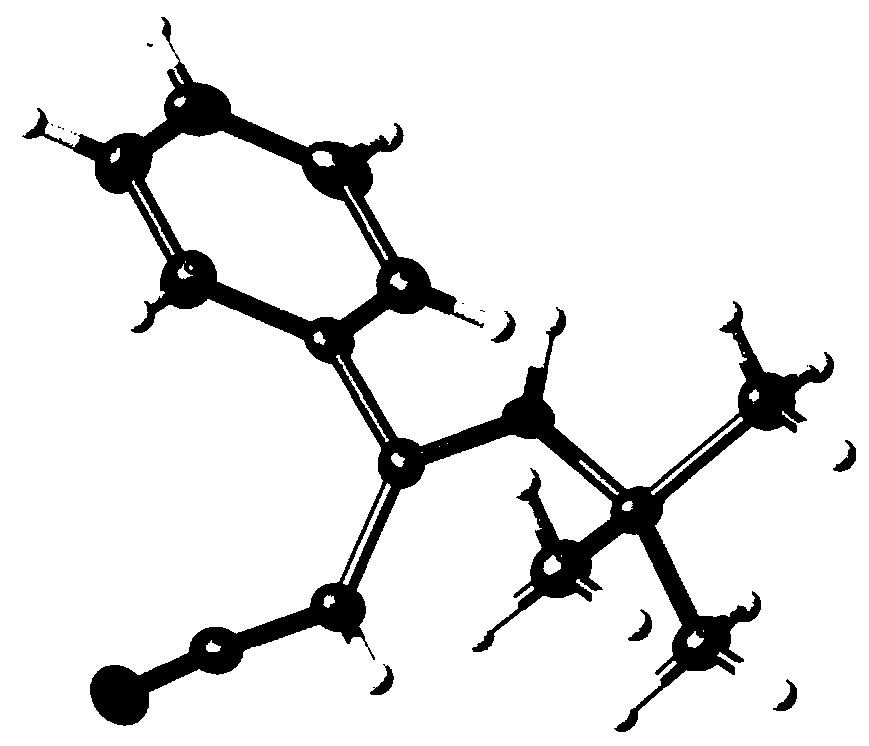
[0040] Its single crystal structure is figure 1 shown. 1 H NMR (300MHz, DMS...
Embodiment 2
[0041] Embodiment 2, the preparation of (E) 3-((4-methoxyphenyl) amino)-3-cinnamonitrile (n)
[0042]Add β-aminocyanoacrylate (8mmol, 1eq), lithium chloride (16mmol, 2eq), dimethyl sulfoxide (40mL), water (10mL) in sequence into a 100mL three-necked flask as shown in formula n-1 , stir to dissolve, and heat to 160°C for 12 hours until the reaction of the raw materials is complete. After cooling to room temperature, 100 mL of ethyl acetate was added, and the organic phase was washed with (2×50 mL) water and 50 mL of saturated saline solution respectively. After precipitation under reduced pressure, the crude product was recrystallized with petroleum ether: ethyl acetate = 4:1 to obtain 1.52 g of gray solid (E) 3-((4-methoxyphenyl)amino)-3-cinnamonitrile (n in Table 1, formula n), yield 76.0%.
[0043]
[0044] product through 1 H NMR, 13 C NMR and MS confirmed:
[0045] 1 H NMR (300MHz, DMSO-d 6 )δ8.80(s,1H),7.63(m,2H),7.58–7.49(m,3H),7.24–7.13(m,2H),7.00–6.91(m,2H),4....
Embodiment 3
[0046] Embodiment 3, the preparation of (E) 3-(tert-butylamino)-3-phenylacrylonitrile (j)
[0047] Add β-aminocyanoacrylate (8mmol, 1eq), lithium chloride (16mmol, 2eq), dimethyl sulfoxide (40mL), water (10mL) in sequence into a 100mL three-necked flask as shown in formula j-1 , stir to dissolve, and heat to 160°C for 12 hours until the reaction of the raw materials is complete. After cooling to room temperature, 100 mL of ethyl acetate was added, and the organic phase was washed with (2×50 mL) water and 50 mL of saturated saline solution respectively. After precipitation under reduced pressure, the crude product was recrystallized with petroleum ether: ethyl acetate = 4:1 to obtain 1.3 g of yellow solid (E) 3-(tert-butylamino)-3-phenylacrylonitrile (j in Table 1, formula j), yield 81.0%.
[0048]
[0049] The product has been single crystal, 1 H NMR, 13 C NMR and MS confirmed:
[0050] Its single crystal structure is figure 1 shown. 1 H NMR (300MHz, DMSO-d 6 )δ7.53–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com