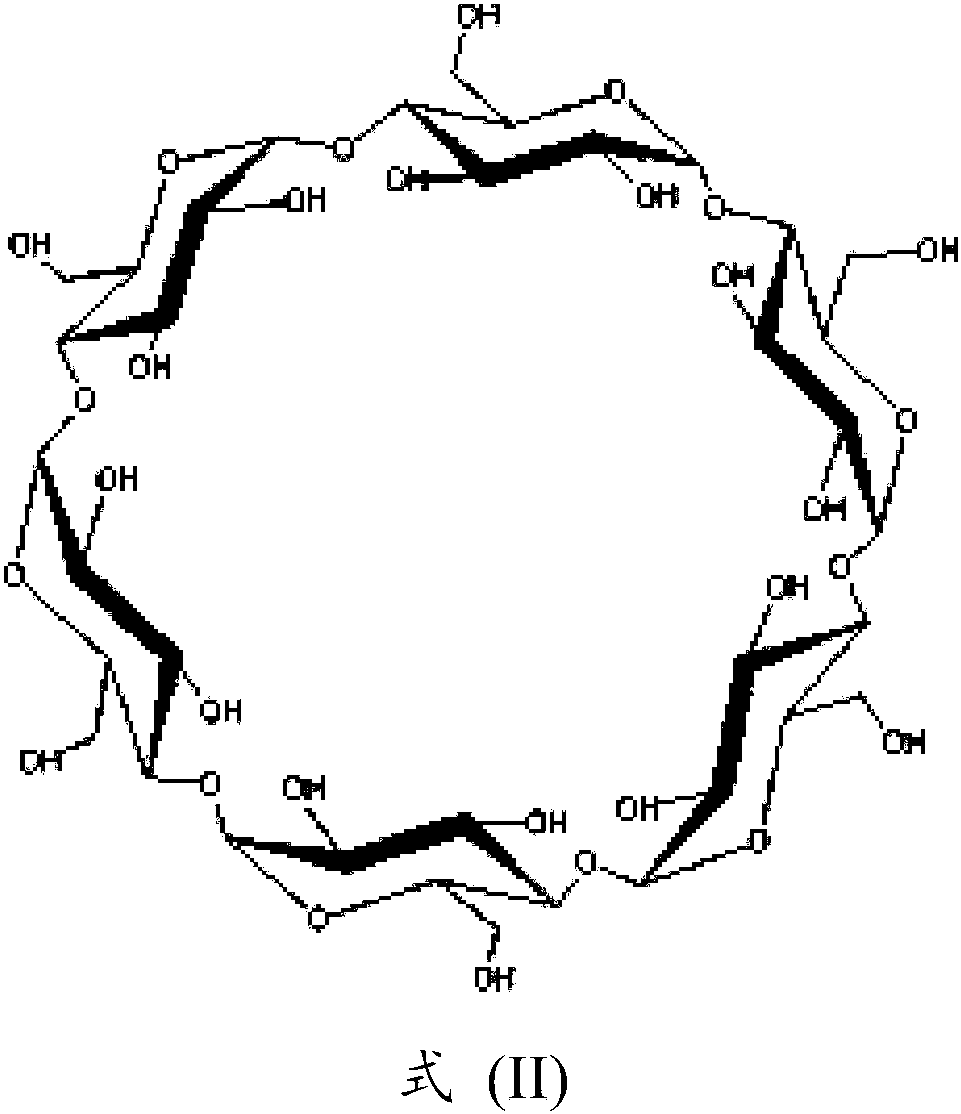
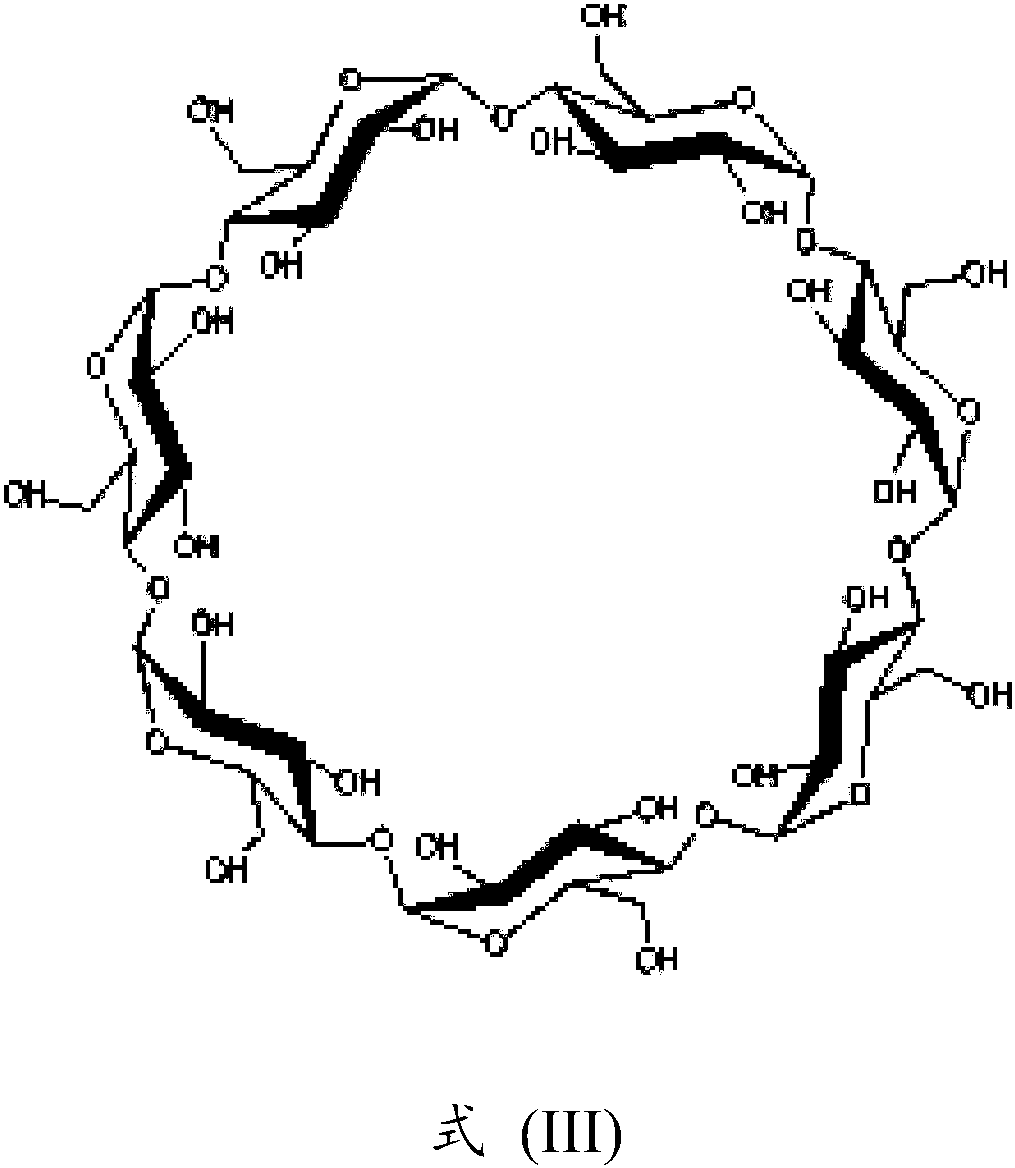
Parenteral formulation comprising siponimod
A parenteral and preparation technology, which is applied in the field of cyprinimod pharmaceutical preparations, can solve problems such as insufficient long-term clinical research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0119] Example 1: Parenteral formulations
[0120] Transfer 250 mL of milliQ water to a suitable glass bottle, then add 50 g of hydroxypropyl beta-cyclodextrin. The mixture was stirred at 500 rpm for 30 minutes, forming a clear solution. Add 556 mg (accurately weighed) of 1-{4-[(1E)-N-{[4-cyclohexyl-3-(trifluoromethyl)benzyl]oxy}ethylimidoyl]-2-ethane benzyl}-3-azetidinecarboxylic acid / fumaric acid (2:1) co-crystal, and the mixture was stirred at 500 rpm for 15 minutes to form a suspension. 305 mg of 2-amino-2-(hydroxymethyl)propane-1,3-diol (Tris, trometamol) was added and the mixture was stirred at 500 rpm for 60 minutes, forming a clear solution with a pH of 7.897. Addition of 250 μl of 1 N NaOH, after stirring at 500 rpm for 2 minutes, resulted in a clear solution with a pH of 7.983. 15 g of mannitol was added and the mixture was stirred at 500 rpm for 15 minutes to form a clear solution. MilliQ water was added to give a clear solution with a pH of 8.015 in a volume of...
example 2
[0122] Example 2: Parenteral formulations
[0123] Transfer 250 mL of milliQ water to a suitable glass bottle, then add 50 g of hydroxypropyl beta-cyclodextrin. The mixture was stirred at 500 rpm for 30 minutes, forming a clear solution. Add 556 mg (accurately weighed) of 1-{4-[(1E)-N-{[4-cyclohexyl-3-(trifluoromethyl)benzyl]oxy}ethylimidoyl]-2-ethane benzyl}-3-azetidinecarboxylic acid / fumaric acid (2:1) co-crystal, and the mixture was stirred at 500 rpm for 15 minutes to form a suspension. 305 mg of 2-amino-2-(hydroxymethyl)propane-1,3-diol (Tris, trometamol) was added and the mixture was stirred at 500 rpm for 60 minutes, forming a clear solution with a pH of 7.878. Addition of 250 μl of 1 N NaOH, after stirring at 500 rpm for 2 minutes, resulted in a clear solution with a pH of 7.997. 3 g of sodium chloride was added, and the mixture was stirred at 500 rpm for 15 minutes, forming a clear solution with a pH of 8.112. Addition of 220 μl of 1 N HCl, after stirring at 500 r...
example 3
[0125] Example 3: Parenteral formulations
[0126]
[0127] 1 This composition refers to a nominal volume of 3.5 mL; an overfill of 0.3 mL is not considered.
[0128] 2 1.112 mg / mL of cipnimod / fumaric acid co-crystal corresponds to 1 mg / mL of cipnimod free base.
[0129] 3 Added as a 1N solution
[0130] 4 Packaging material: (i) vial R 6ML / 20MM amber, (ii) STPF LYO CIIR 20MM D21-7S / V10-F322-2W / RS (grey rubber stopper), (iii) AR-KAPPE PP / AL 20MM NATUR / NATUR (aluminum cover with plastic flip disc).
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com