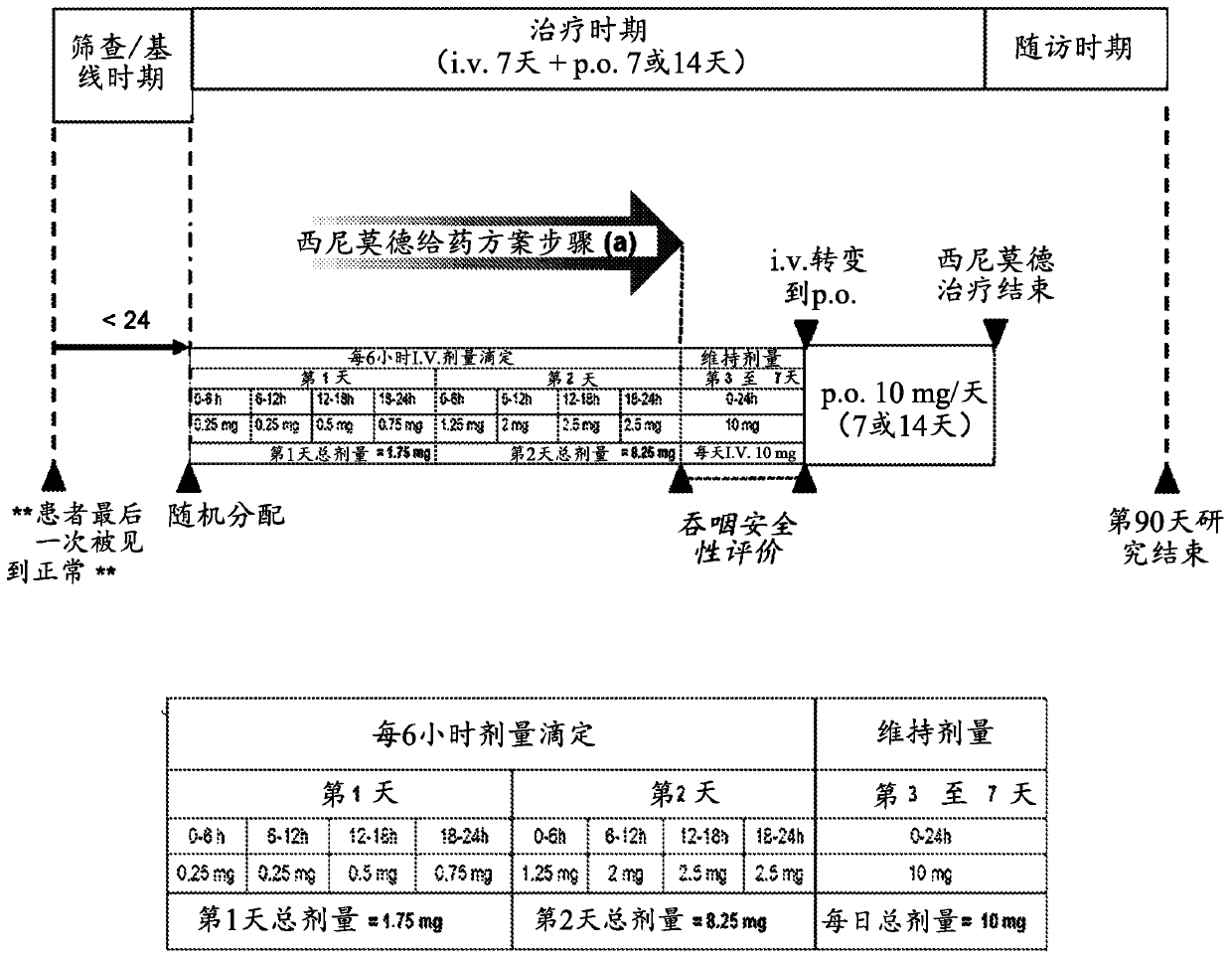
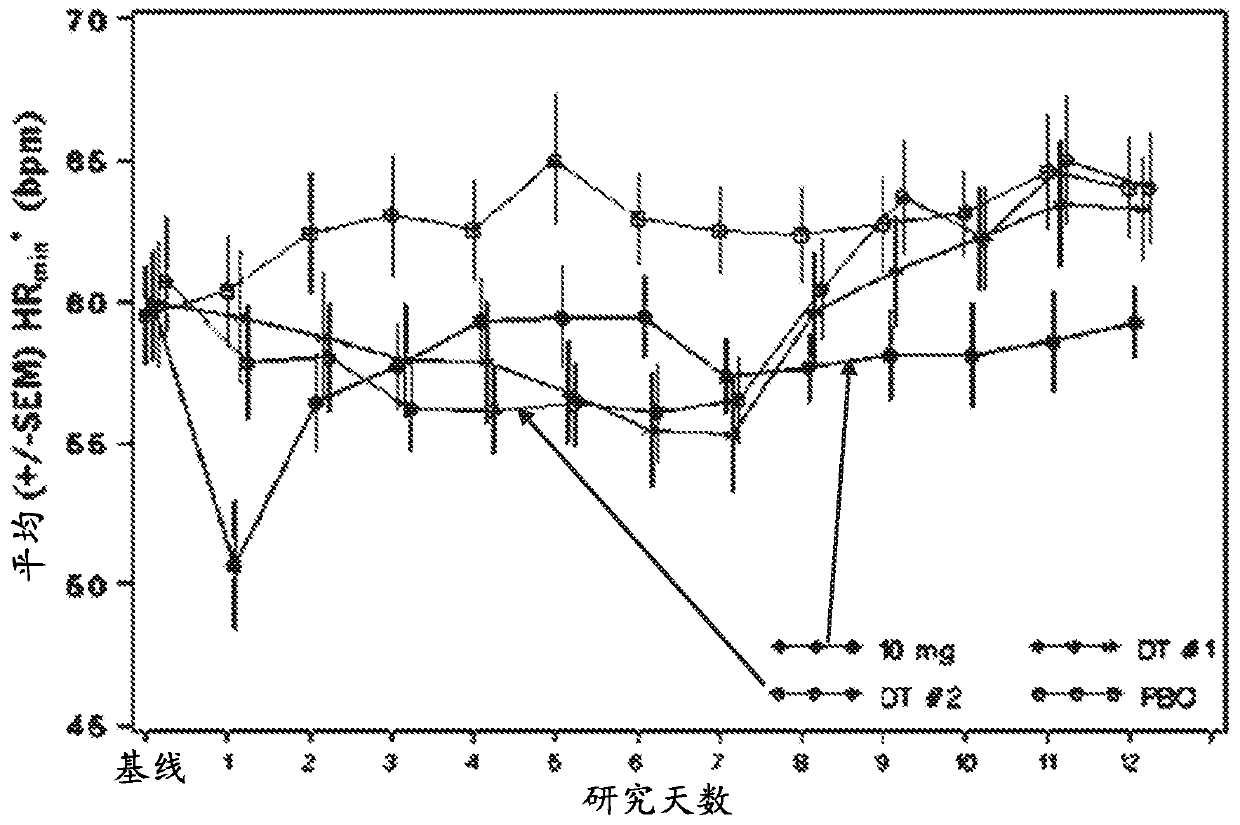
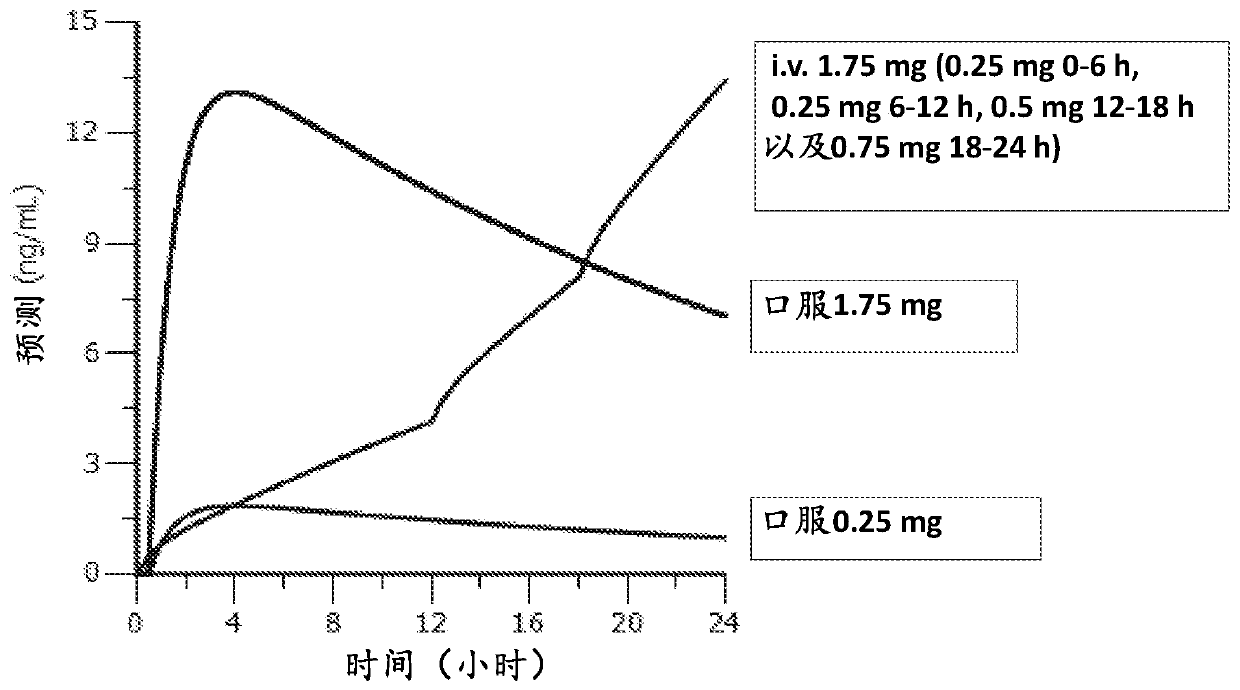
Dosing regimen of siponimod
A dose, stroke technology, applied in the dosing regimen of siponimod, that addresses incompletely understood limitations, unmet high needs, inflammatory and immune mechanisms that are not well understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0091] Embodiment 1.1: A method of treating stroke in a human subject suffering from stroke, the method comprising:
[0092] (a) administering multiple consecutive doses of sinimod intravenously to the subject within a period of time equal to or at most 96 hours from the first intravenously administered dose, wherein
[0093] (i) the first administered dose is not less than 0.25 mg and not more than 1.25 mg;
[0094] and among them
[0095] (ii) each of one or more consecutive doses administered after said first dose is no less than the immediately preceding dose and no more than the subsequent directly administered dose;
[0096] and among them
[0097] (iii) the sum of said consecutive doses administered over a consecutive 24-hour period is less than the daily maintenance dose; and subsequently
[0098] (b) administering a daily maintenance dose of sinimod for a maintenance period of at least 2 days, wherein
[0099] (i) The daily maintenance dose is not less than 2 mg a...
Embodiment 12
[0100] Embodiment 1.2: The method of treating stroke in a human subject as defined in embodiment 1.1, wherein said subject is administered intravenously to said subject according to step (a) said multiple consecutive doses of sinimod after the within a period equal to or at most 72 hours from the start of the first intravenously administered dose.
Embodiment 13
[0101] Embodiment 1.3: A method of treating stroke in a human subject as defined in Embodiment 1.1 or 1.2, wherein said subject is administered intravenously to said subject according to step (a) said multiple consecutive doses of sinimod at within a period equal to or at most 48 hours from the first intravenously administered dose.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com