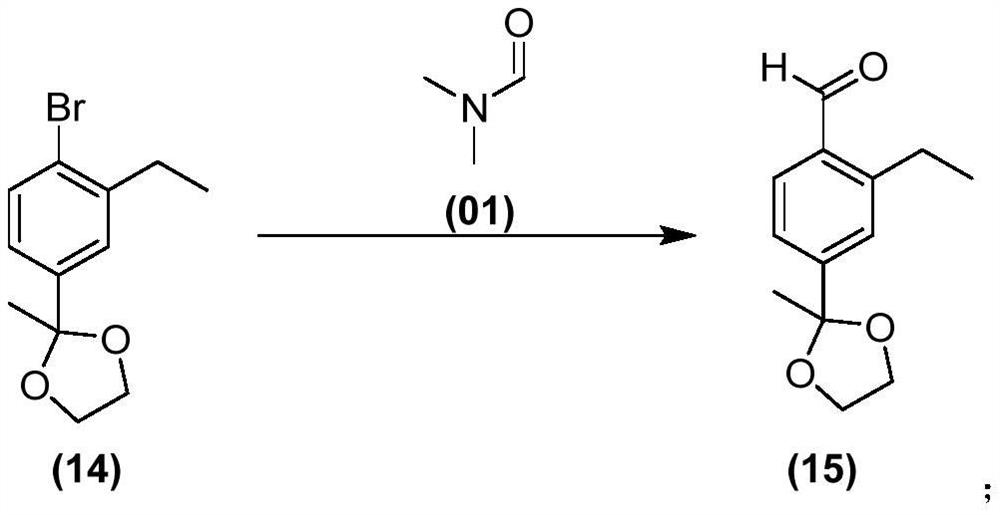
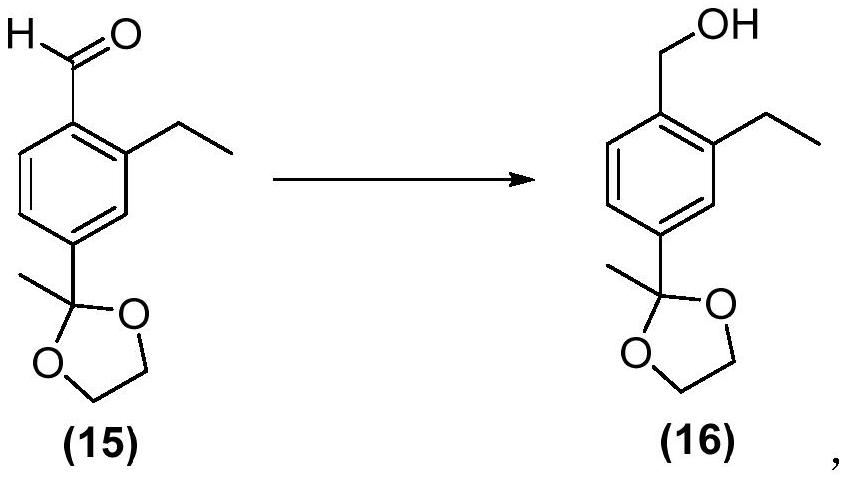
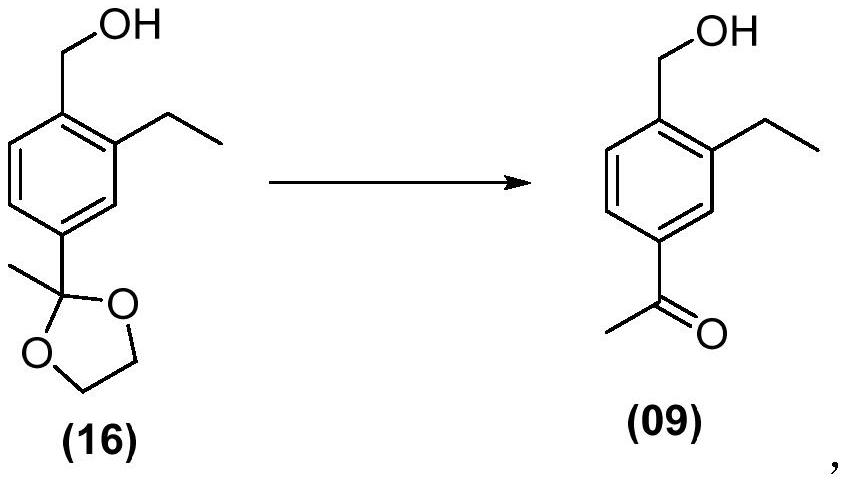
Siponimod intermediate and preparation method thereof
A compound and reaction solvent technology, applied in the field of siponimod intermediate and its preparation, can solve the problems of limited industrial application, high cost, and low reaction safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation of embodiment 1 compound (10)
[0075]
[0076] Add compound (9) (60.59g), dichloromethane (480mL), 4-dimethylaminopyridine (134.39g), di-tert-butyl dicarbonate (272.81g) into a 1000mL three-necked reaction flask, and heat up to 40°C After stirring for 4h, water (240mL) was added after the reaction was completed, and the organic phase was concentrated under reduced pressure after liquid separation to obtain compound (10) (160.71g). The crude product yield was 100%, and the purity: 95.2%; .
Embodiment 2
[0077] The preparation of embodiment 2 compound (12)
[0078]
[0079] Add dichloromethane (200mL) into a 1000mL three-necked reaction flask, cool down to 0°C, add aluminum trichloride (29.33g), dropwise add acetyl chloride (15.70g), and dropwise add compound (10) (64.28g ) in dichloromethane (200mL). After the dropwise addition, control the temperature and react at 25°C for 3 hours. After the reaction, drop the reaction solution into dilute hydrochloric acid (2mol / L, 300mL). After the dropwise addition, control the temperature and react at 25°C for 2h. After the reaction, add hydrogen The sodium oxide aqueous solution was adjusted to pH ≈ 9-12, and the aqueous phase was extracted once with dichloromethane (200mL) after separation, the combined organic phase was washed with water (200mL), the organic phase was concentrated under reduced pressure to obtain a yellow oil, and then washed with ethanol Recrystallization (10vol absolute ethanol, heated to 70°C for dissolution, c...
Embodiment 3
[0081] The preparation of embodiment 3 compound (13)
[0082]
[0083] Add 48% hydrobromic acid aqueous solution (50mL) and compound (12) (16.32g) into a 500mL three-necked reaction flask, cool down to 0°C, control the temperature at 0-5°C and slowly add sodium nitrite (6.90g). Add the above reaction solution to 20% hydrobromic acid solution (20mL) containing cuprous bromide (20.08g), heat up to reflux temperature after addition, react for 5h, cool down to room temperature after the reaction, and dichloromethane Methane (100mL) was extracted three times, the combined organic phase was washed with water (150mL), the organic phase was concentrated under reduced pressure to obtain a yellow oil, and then column chromatography (gradient elution, n-hexane:ethyl acetate=10:1 to 4 :1) A light yellow solid (18.64g) was obtained, yield 82.1%, purity: 96.8%; MS: [M+1]=227.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com