Salmonella abortus equi horse derived strain and application thereof in preparation of salmonella equina inactivated vaccine
A technology of salmonella and inactivated vaccines, which is applied in the direction of vaccines, veterinary vaccines, bacteria, etc., can solve the problems of spreading, affecting the stock of horses, indirect economic losses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
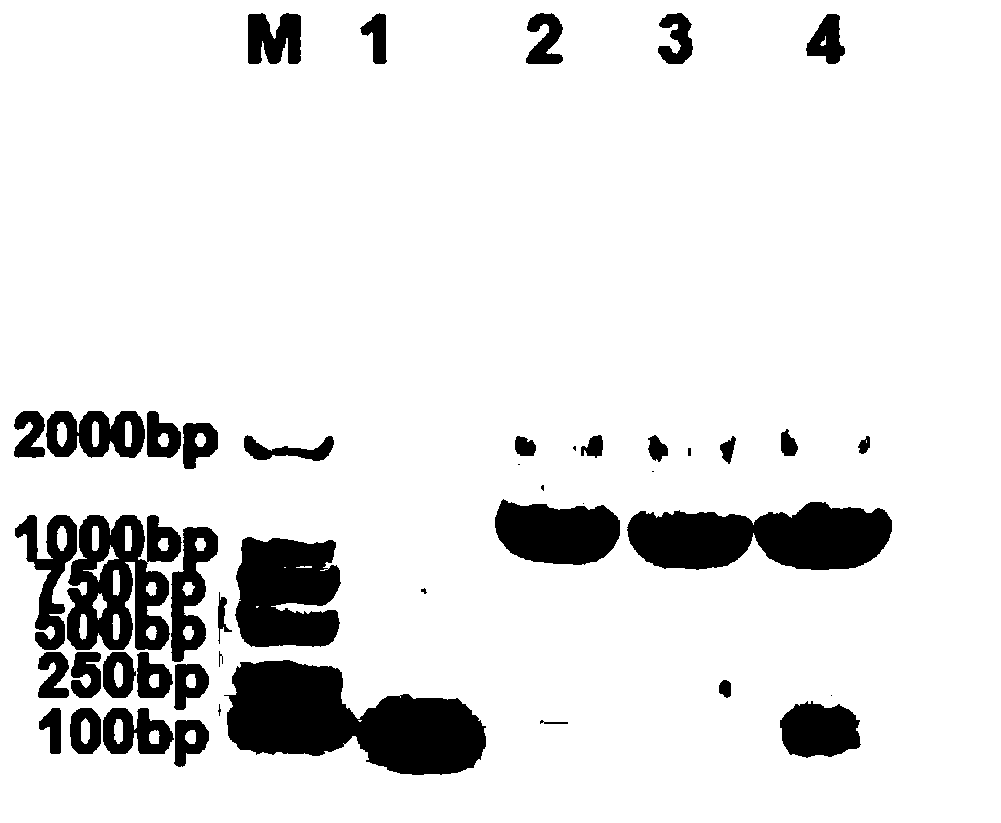
[0032] Example 1 Isolation and culture identification of Salmonella equine abortus strain 20180316.H.AES.G
[0033] According to the new biological product declaration requirement, in conjunction with a large amount of test data that the present invention obtains, with reference to " Chinese Veterinary Pharmacopoeia " (2005 edition), the poisonous species used for making seedlings has been identified, and the poisoned species standard of the trial procedure (draft) is now carried out as follows illustrate.
[0034] 1 Isolation and identification of virus species
[0035] The virus species used to manufacture this product is Salmonella equine abortifaciens equine source strain 20180316.H.AES.G, which was isolated from an aborted foal tissue by the inventor of the present invention in 2018 from a horse farm in Hulunbeier City, Inner Mongolia. Salmonella abortus.
[0036] The liver tissue of the aborted foal was streak-inoculated on the Salmonella chromogenic medium, and the Sa...
Embodiment 2
[0052] The preparation of embodiment 2 inactivated vaccines
[0053] 1 inactivation process
[0054] Choose formaldehyde solutions with final concentrations of 0.1% v / v, 0.15% v / v, and 0.2% v / v to inactivate at 4, 28°C, and 37°C, and inactivate 0.2% formaldehyde at 28°C for 48 hours, and the inactivation effect is complete . Therefore, it is finally determined that 0.2% v / v formaldehyde is inactivated at 28°C for 48 hours, coated with no anti-LB plate, cultured at 37°C for 3 days, and sterile, then the inactivation is qualified, and then the inactivation is continued for 12 hours. Wash 2 times with PBS to remove formaldehyde.
[0055] 2 emulsification process
[0056] Commercialized MONTANIDE ISA35 adjuvant from Sebec, France, and 0.2% v / v formaldehyde (HCHO) inactivated Salmonella equine abortus strain 20180316.H.AES.G were mixed and emulsified to make inactivated vaccine, adjuvant The concentration is 25%. Make up to volume with PBS.
[0057] 3 Quality standards for in...
Embodiment 3
[0078] Embodiment 3 horse safety test
[0079] 3 batches of inactivated vaccines qualified by the safety test prepared in Example 2 were immunized to stallions, non-pregnant and pre-pregnancy, mid-pregnancy, late-pregnancy mares (10 billion / ml / horse, 1.0 × 10 10 CFU / ml), observe whether the experimental horse absorbs the vaccine, whether the diet, drinking water, and mental state are normal. The results showed that only a few horses did not like to eat grass, drinking water was normal, and some horses had swelling at the immune site, but no ulceration. Generally, the swelling subsided on the 4th day, and the swelling disappeared completely within 2 weeks. Individual body temperature rises briefly and returns to normal within 2 days. After one month of observation, vaccine immunization will not cause abortion in pregnant mares, as shown in Table 5.
[0080] Table 5 Horse body safety test
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com