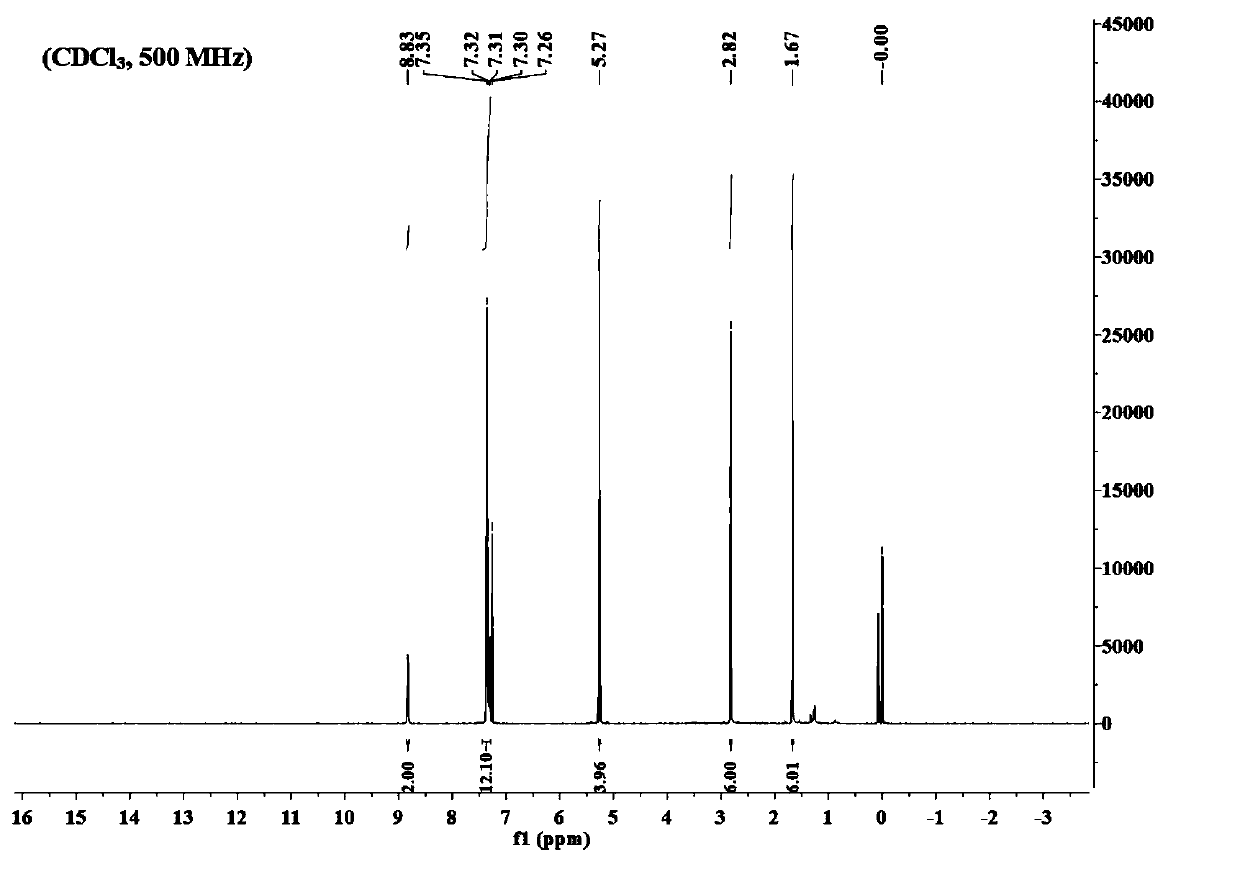
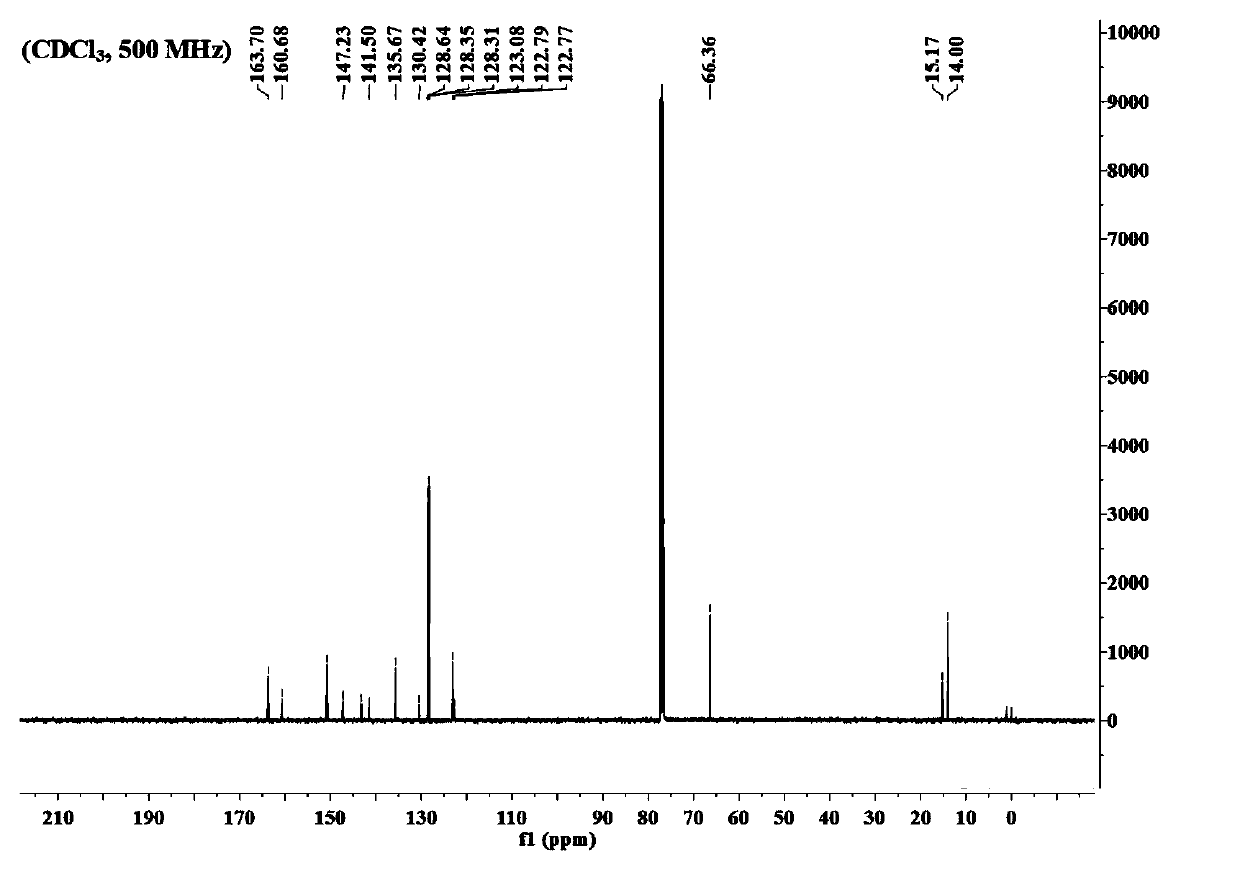
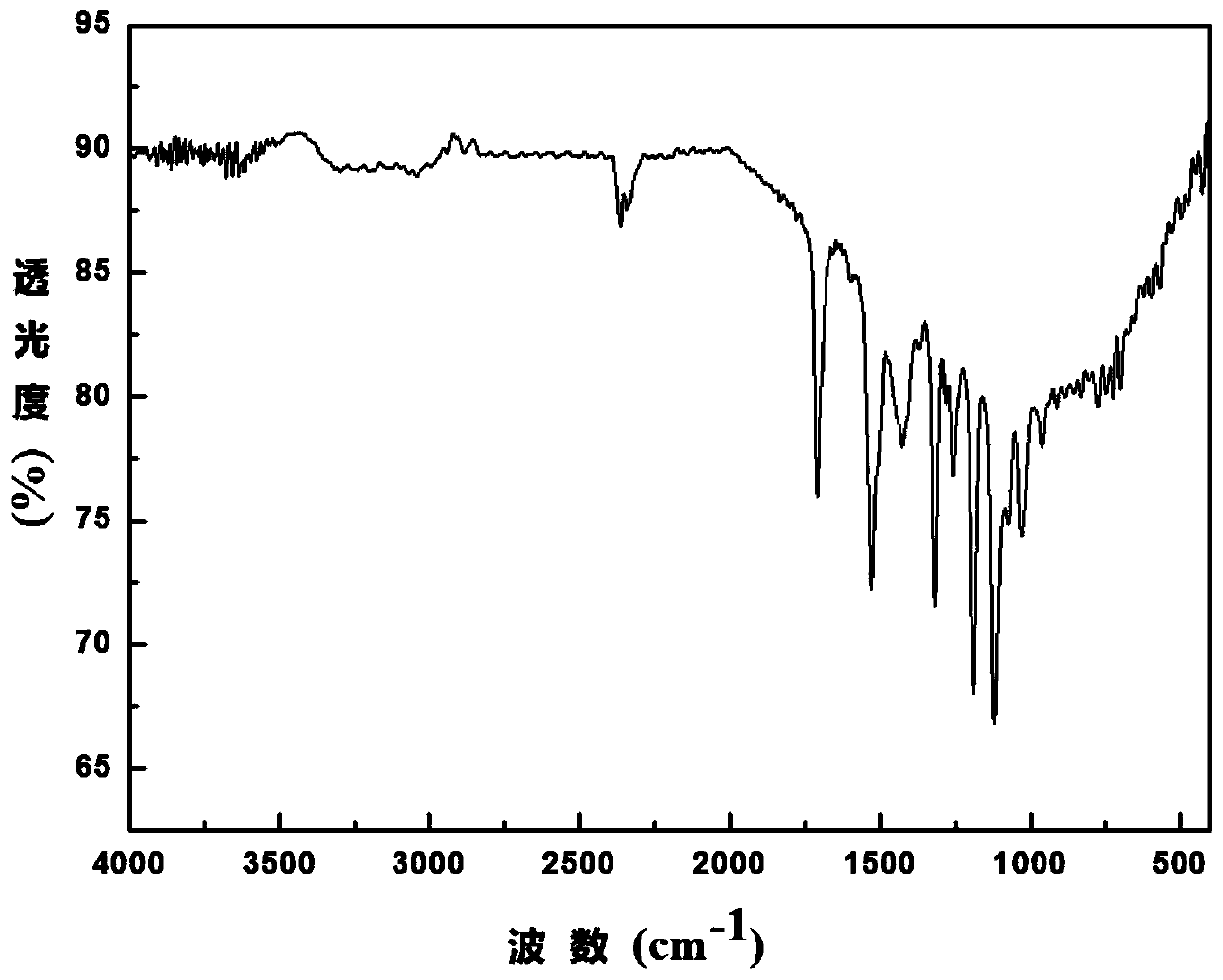
Boron dipyrromethene derivative dye ligand and preparation method thereof
A technology for fluoroboron dipyrrolemethine and derivatives is applied in the field of fluoroboron dipyrrolemethine derivative dye ligands and their preparation, and achieves the effects of simple and easy-to-control methods, easy-to-obtain raw materials and universal adaptability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of a fluoroborate dipyrromethene derivative dye ligand, comprising the following steps:
[0046] Step 1. First, under magnetic stirring, add 2,4-dimethyl-1H-pyrrole-3-methyl benzyl (800mg, 3.5mmol) and 4-pyridinecarbaldehyde ( 200 μL, 1.97 mmol) and dry dichloromethane (200 mL), then dropwise added 200 μL trifluoroacetic acid (1.08 mmol) under a helium atmosphere, and reacted at 25 ° C for 20 h in the dark to obtain a reaction solution A;
[0047] Step 2. Add 380mg of 2,3-dichloro-5,6-dicyano-p-benzoquinone (1.67mmol) dropwise to the reaction solution A obtained in step 1 under stirring for oxidation reaction, and stir the reaction at 25°C for 4h, Obtain reaction solution B;
[0048] Step 3. Slowly add 6 mL of N,N-diisopropylethylamine (36.3 mmol) to the reaction solution B obtained in Step 2, and then dropwise add 9 mL of boron trifluoride-diethyl ether solution in an ice bath (0°C) (71mmol) to react, continue to stir and react for 8h, after thin...
Embodiment 2
[0053] A preparation method of a fluoroborate dipyrromethene derivative dye ligand, comprising the following steps:
[0054] Step 1. First, under magnetic stirring, add 2,4-dimethyl-1H-pyrrole-3-methyl benzyl (798.7mg, 3.5mmol) and 4-pyridinecarbaldehyde prepared according to the reference to a 500mL three-necked flask in sequence. (220μL, 2.2mmol) and dry dichloromethane (100mL), then add 200μL trifluoroacetic acid (1.08mmol) dropwise under a helium atmosphere, and react at 30°C in the dark for 40h to obtain a reaction solution A;
[0055] Step 2. Add 382.2mg of 2,3-dichloro-5,6-dicyano-p-benzoquinone (1.68mmol) dropwise to the reaction solution A obtained in step 1 under stirring for oxidation reaction, and stir the reaction at 30°C for 40h , to obtain reaction solution B;
[0056] Step 3. Slowly add 6mL of N,N-diisopropylethylamine (36.3mmol) to the reaction solution B obtained in step 2, and then add 9mL of boron trifluoride-ether solution (71mmol) dropwise at 25°C. Reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com