Sesquiterpenoid compound, fungus secondary metabolite extract containing sesquiterpenoid compound and application of sesquiterpenoid compound
A technology of secondary metabolites and sesquiterpenoids, applied in the field of medicine, can solve problems such as lipase inhibition that has not been seen yet, and achieve good research and development prospects and high yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of fungal secondary metabolite extract comprises the steps:
[0027] (1).
[0028] Preparation of strain liquid seed solution: The formula of the culture solution is 200g of potatoes, 10g of yeast extract, 6g of peptone, 30g of glucose, KH 2 PO 4 2g, MgSO 4 0.5g, dilute to 1L, adjust pH to 6.8. Pack into 500mL Erlenmeyer flasks, fill each bottle with about 150mL, sterilize at 115°C, 68kPa for 30 minutes. After being inoculated with the Penicillium purpurogenum IMM003 strain, culture it for 3 days at 28°C and 155 rpm. Then the culture solution was diluted 100 times and used as seed solution for future use.
[0029] (2) Strain solid fermentation: put 150g rice and 180mL purified water into a 1L Erlenmeyer flask, sterilize at 115°C, 68kPa for 30 minutes, inoculate Penicillium purpurogenum IMM003 seed solution, and cultivate at 28°C for 40 days. After soaking in 500 mL of ethyl acetate for 30 minutes, it was extracted three times by ultrasonic extrac...
Embodiment 2
[0031] The preparation of sesquiterpene compound comprises the steps:
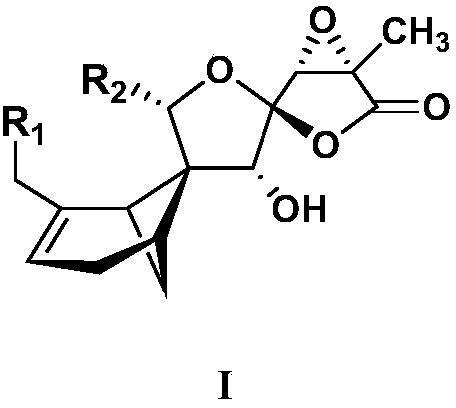
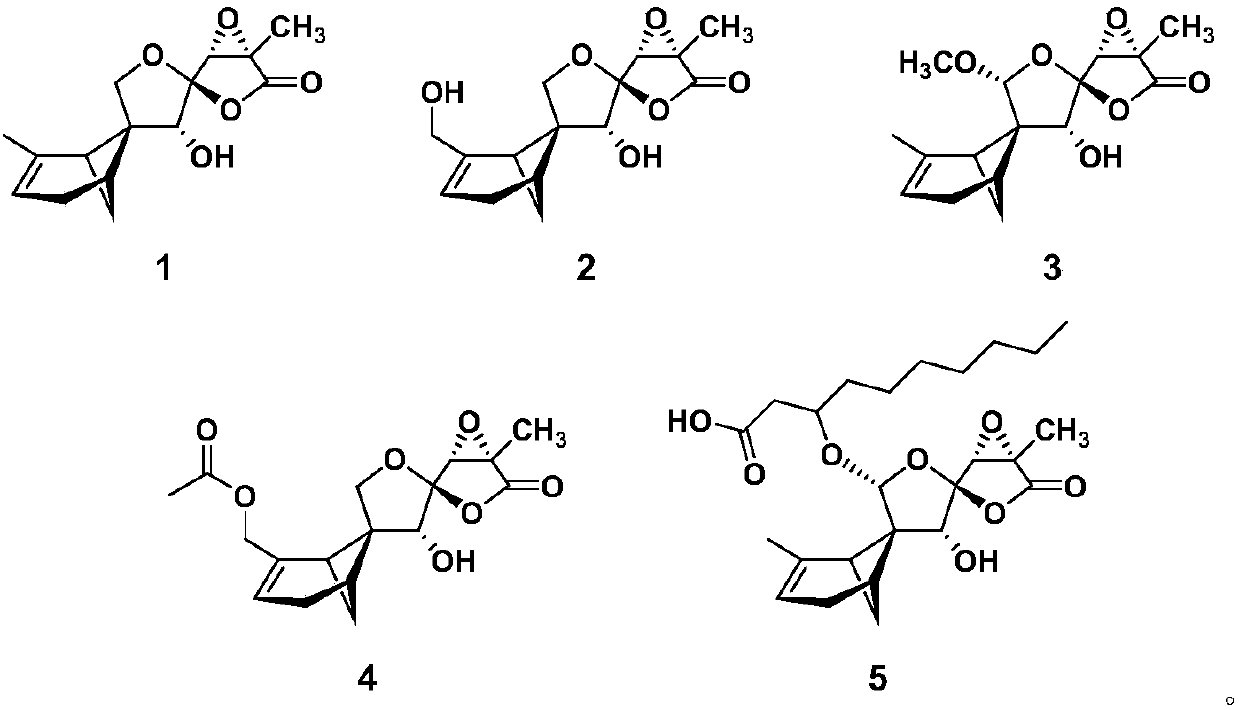
[0032] The extract obtained in Example 1 was eluted by medium-pressure silica gel column chromatography (petroleum ether-acetone 50:1, 20:1, 5:1, 3:1, 0:1) to obtain components A-F. Select component B and then normal pressure silica gel column chromatography (petroleum-ether acetone 100:1, 50:1, 20:1, 10:1, 7:1, 5:1, 3:1, 2:1, 1:1 , acetone, methanol) to obtain component B1-11. Component B5 via Flash C 18 Column chromatography (20%, 30%, 50%, 70%, 90% methanol-water gradient elution) yielded components B5-1 to B5-5. B5-1 was purified by Sephadex LH-20 column chromatography to obtain compound 1 (45.0 mg). Component B7 via Flash C 18 Column chromatography (20%, 30%, 50%, 70%, 90% methanol-water gradient elution) yielded components B7-1 to B7-5. Component B7-1 was separated by HPLC semi-preparative chromatography (ES-C18 column, 75% methanol-water, 205 nm) to obtain compound 3 (19 min, 6.0 mg). B7-2 was...
experiment example 1
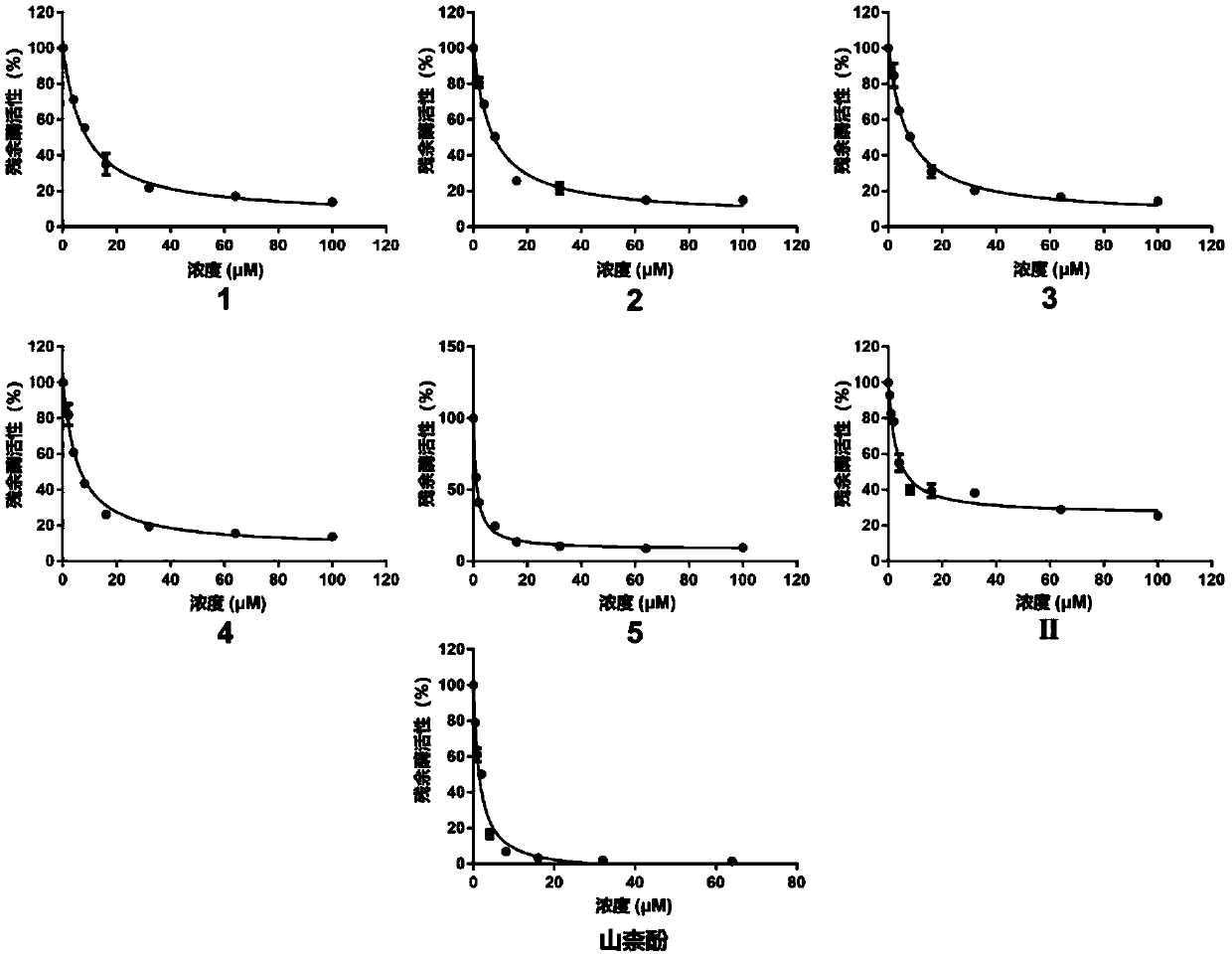
[0048] Experimental example 1: Evaluation of the inhibitory effect of fungal secondary metabolite extracts and compounds on pancreatic lipase activity
[0049] Instruments: metal incubator, vortex shaker, microplate reader
[0050] Reagent: including 0.1M Buffer (citric acid-disodium hydrogen phosphate) buffer (pH 7.4), 0.25mM 4-MUO (4-methylumbelliferone) and 1mg / ml lipase (dissolved in buffer)
[0051] Experimental steps:
[0052] 1) The microsome incubation system contains the following components (total volume 200μl):
[0053]
[0054] Table 3 lipase incubation system and addition amount
[0055]
[0056] 2) Add lipase+buffer+Inhibitor / DMSO to a black 96-well plate, shake and mix well, then place in a metal bath / water bath at 37°C for 10 minutes for pre-incubation;
[0057] 3) After adding 2 μl 4-MUO to start the reaction, perform fluorescence analysis, that is, kinetic detection with a fluorescent microplate reader, and the time is 30 minutes;
[0058] 4) Set up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com