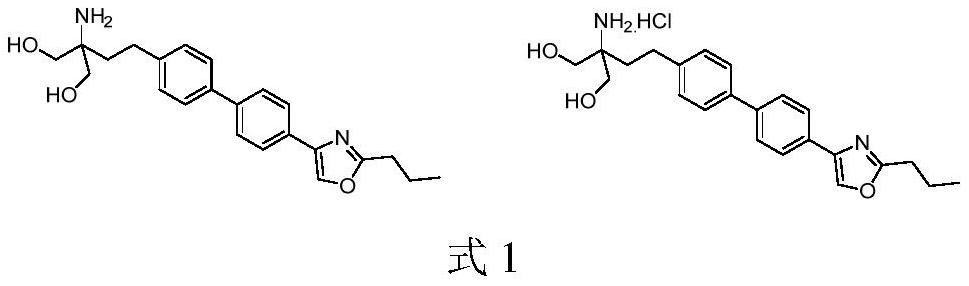
The preparation method of Prosymod
A synthesis method and intermediate technology, applied in the field of medicine, can solve the problems of low yield, long synthesis route, large environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
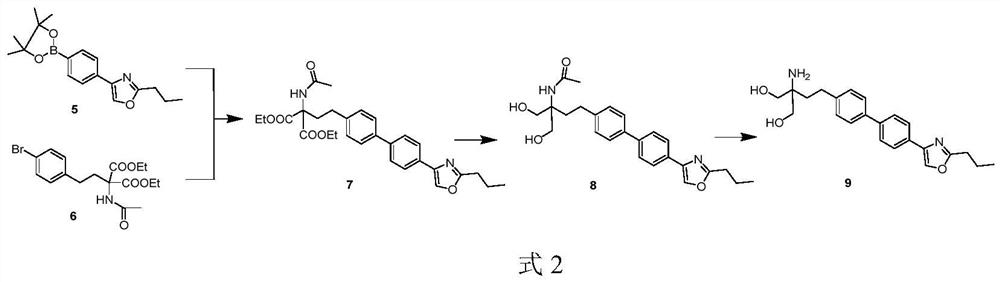
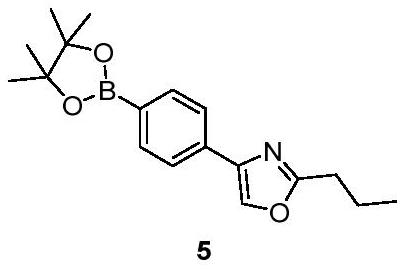
[0016] The technical scheme that realizes the object of the present invention is to provide the new preparation method of Procymod, and the synthetic steps are as follows: the preparation route of the key intermediate 5 is shown in formula 3, starting from bromobenzene, the first step is to pass the bromobenzene through the pyridoxyl group. In the second step, compound 2 is esterified with butyrate to form compound 3, and in the third step, compound 3 is cyclized with butyramide to form compound 4. In the fourth step of reaction, compound 4 is coupled with double pinacol boronate under the catalysis of palladium catalyst to generate compound 5.
[0017]
[0018] a. Dissolve bromobenzene (compound 1) and chloroacetyl chloride in anhydrous dichloromethane, add anhydrous alumina in batches under an ice bath, and finish the reaction in about 0.5-3 hours. The reaction solution was poured into a 1 mol / L hydrochloric acid ice-water mixture. Extraction, drying, and solvent removal...
preparation example 1
[0043] 2-propyl-4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)phenyl)oxazole (Compound 5) Synthesis
[0044]
[0045] a. Synthesis of 1-(4-bromophenyl)-2-chloro-1-one (compound 2): Dissolve 20.0 g of bromobenzene in a solution of dichloromethane (200 mL) and place it in a three-necked flask to cool at external temperature Under stirring, 10.6 mL of chloroacetyl chloride was dissolved in dichloromethane (50 mL) and slowly dropped into a three-necked flask. Keeping the low temperature, 20.4 g of anhydrous aluminum chloride was added to the reaction flask in three batches (7.0 g for the first time, 7.0 g for the second time, and 6.4 g for the third time). After 1 hour, the reaction was basically completed, and the reaction solution was poured into 250 mL of dilute hydrochloric acid-ice-water mixture and stirred for half an hour until the reaction became light yellow-green. Dichloromethane was used for extraction and separation (200 mL × 3), washed with saturated brine (2...
preparation example 2
[0050]
[0051] Synthesis of 2-acetamido-2-(4-bromophenethyl) diethyl malonate (key intermediate 6):
[0052] a. Synthesis of 1-bromo-4-(2-iodoethyl)benzene (compound 11): 31.6 g of iodine, 33.9 g of triphenylphosphine and 22.4 mg of imidazole were dissolved in anhydrous dichloromethane (100 mL), After cooling in an ice bath for 30 minutes, 20.0 g of p-bromophenethyl alcohol was dissolved in anhydrous dichloromethane (50 mL), and quickly added dropwise to the reaction flask. The ice bath was removed, and the reaction was carried out at room temperature for about 6 hours. Dichloromethane was evaporated, water-methanol mixed solution (400mL, the volume ratio of water:methanol was 1:3) was added to a separatory funnel, n-heptane was added for extraction (200mL×3), and the whole system was shaken vigorously The color is transparent, and the organic layers are combined. It was dried over anhydrous sodium sulfate, and the organic solvent was evaporated to obtain a colorless and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com