A multi-channel mass spectrometry derivatization reagent for detecting hydroxy polycyclic aromatic hydrocarbons and its preparation method and application
A technology for derivatizing reagents and polycyclic aromatic hydrocarbons, which is applied in the field of multi-channel mass spectrometry derivatizing reagents for detecting hydroxy polycyclic aromatic hydrocarbons and its preparation and application. It can solve the problems of expensive internal standard compounds, long derivatization time, and low analysis throughput. The method has good applicability, simple operation and high analytical throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
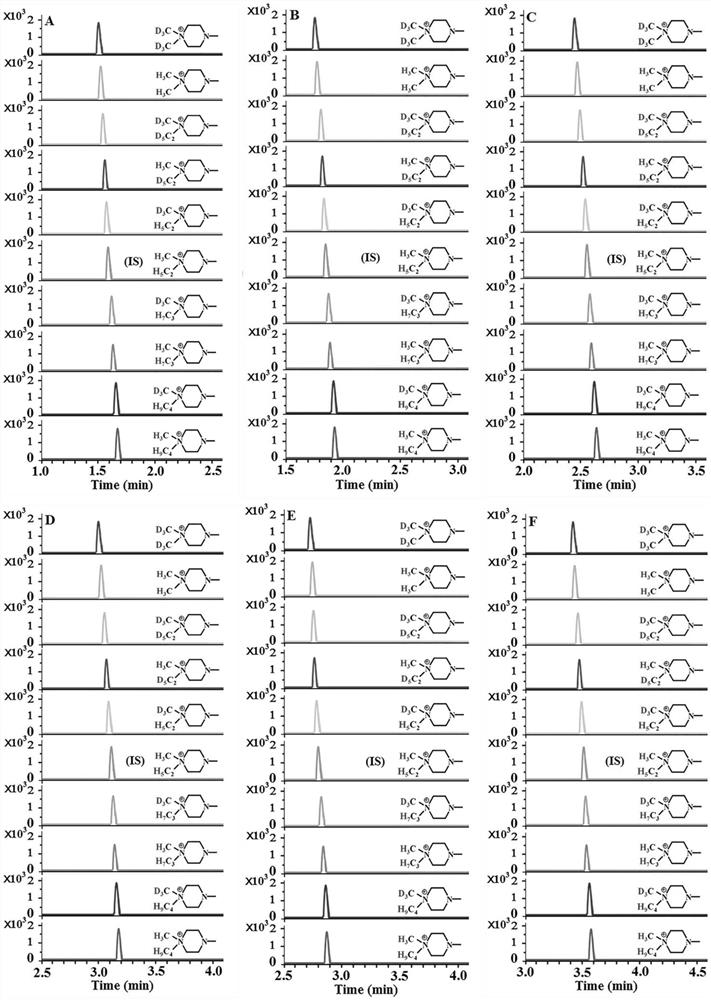
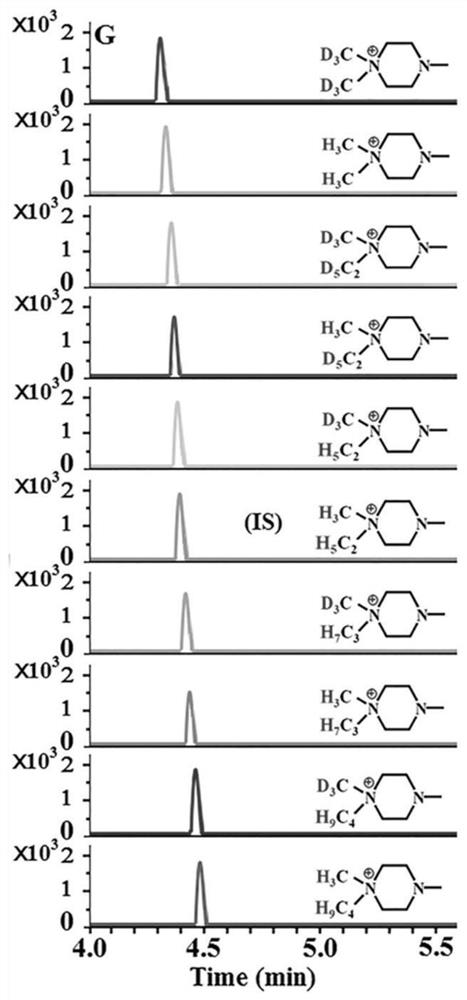
[0049] Chromatographic separation and mass spectrometry qualitative and quantitative analysis of 7 hydroxy polycyclic aromatic hydrocarbons:
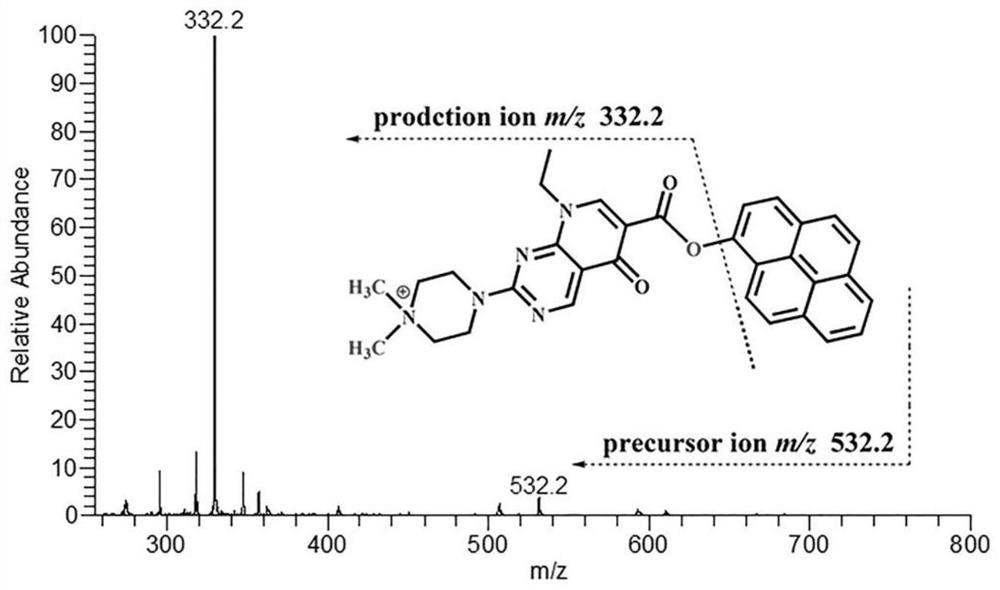
[0050] Seven kinds of hydroxy PAH standard substances (purchased from Sigma, J&K and Aladdin Reagent Company) were prepared in acetonitrile to obtain a concentration of 1.0 × 10 -2 mol / L hydroxy polycyclic aromatic hydrocarbon standard solution. We centrifuged the analyte-free urine at high speed to remove impurities, added acetonitrile (1:3, v / v) to the analyte-free plasma, centrifuged for 5 minutes (12000rpm), separated the supernatant for use, and processed Unanalyzed urine and plasma were mixed in the same proportion as a blank matrix. Take 0.2 mL from each single-standard stock solution and dilute to 10 mL with a blank matrix to obtain a mixed-standard stock solution of hydroxy-PAHs. 4'-(N,N-dialkyl)-1-piperazinyl-6-carbonyl chloride-piperidic acid was dissolved in acetonitrile to obtain 1.0×10 -3 mol / L derivative reagent solution...
Embodiment 2
[0057] The detection and analysis of free hydroxy polycyclic aromatic hydrocarbons in urine includes the following steps:
[0058] Take 500 μL of urine from smokers or non-smokers and centrifuge at high speed to remove impurities, then take 9 parts of 50 μL urine samples and place them in 1.5 mL centrifuge tubes, and take another 50 μL standard mixed solution (1.0×10 -5 mol / L) in a 1.5mL centrifuge tube, add 250μL NaHCO 3 -Na 2 CO 3 (pH 9.8) buffer solution, vortex shaker for 15s, add 550 μL of other 9 kinds of SIMTs (SIMT-332 / 338 / 349 / 351 / 354 / 360 / 363 / 374 / 377) solution, SIMT-346 solution, Seal and react at 40°C for 4.5 minutes. Then the SIMT-346-labeled standard derivatization product solution (internal standard solution) and other 9 kinds of derivatization reagent-labeled urine sample derivatization product solutions were mixed in equal volumes in a 1.0mL test tube (derivatization product mixed solution), and then used for MDSPE program.
[0059] 7 mg of adsorbent (Fe 3 ...
Embodiment 3
[0061] The detection of free hydroxy polycyclic aromatic hydrocarbons in plasma includes the following steps:
[0062] Take 200 μL of plasma from smokers or non-smokers, add 100 μL of acetonitrile, centrifuge at high speed for 5 minutes (12000 rpm) and separate the supernatant for use. Take 9 parts of 20μL plasma samples and place them in 1.5mL centrifuge tubes, and take another 20μL standard mixed solution (1.0×10 -5 mol / L) in a 1.5mL centrifuge tube, add 100μL NaHCO 3 -Na 2 CO 3 (pH 9.0) buffer solution, vortexed for 15s, respectively added 150 μL other 9 kinds of SIMTs (SIMT-332 / 338 / 349 / 351 / 354 / 360 / 363 / 374 / 377) solution, SIMT-346 solution, Seal and react at 40°C for 6 minutes. Then the SIMT-346-labeled standard derivatization product solution (internal standard solution) and other 9 kinds of derivatization reagent-labeled plasma sample derivatization product solutions were mixed in equal volumes in a 1.0mL test tube (derivatization product mixed solution), and then used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com