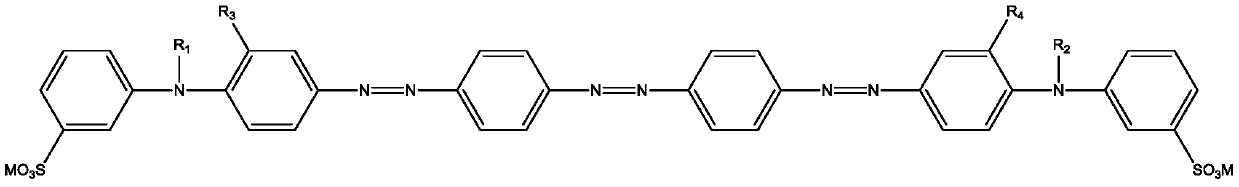
Trisazo carmoisine and preparation method thereof
An acid dye, trisazo technology, applied in trisazo dyes, dyeing, textiles and papermaking, etc., can solve problems such as unenvironmental protection, and achieve the effects of high dyeing rate, good water solubility and good levelness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of embodiment 1, trisazo red acid dye I-1, carries out the following steps successively:
[0044] 1), in the reactor equipped with a stirring device, start stirring, add 200g of water, add 0.1mol (about 21.23g) of p-diaminoazobenzene, add 0.5mol of hydrochloric acid (hydrochloric acid with a mass fraction of 31%), Add ice and cool to 0-5°C, add dropwise sodium nitrite solution containing 0.205 mol of sodium nitrite (sodium nitrite solution with a mass fraction of 30%), and control the temperature of the reaction system during the dropwise addition to not exceed 5°C. When the starch potassium iodide test paper turns blue, react at 0-5°C for 2 hours (the end point is no color detected by Ehrlich reagent), and then use sulfamic acid to destroy the remaining nitrous acid after the reaction (the starch potassium iodide test paper does not turn blue as the end point). Standard, the amount of sulfamic acid is about 0.5g); get diazonium salt solution (all...
Embodiment 2
[0050] The preparation method of embodiment 2, three azo red acid dyes I-2, carries out the following steps successively:
[0051] 1), with the step 1) of embodiment 1;
[0052] 2) In the reactor equipped with stirring, start the stirring, add 400g of water, add 0.202mol of N-ethyl-N-benzyl m-toluidine-3'-sulfonic acid, add soda ash to adjust the pH=7.0~8.0, Stir until the N-ethyl-N-benzyl-m-toluidine-3'-sulfonic acid dissolves.
[0053]Add ice to cool to 0-5°C, slowly add all the product (diazonium salt solution) obtained in step 1) dropwise, control the temperature of the reaction system not to exceed 10°C during the dropwise addition, and adjust the pH to 7.0 with soda ash during the dropwise addition ~8.0, after adding, react at 5~10℃ for 3 hours, add sodium chloride (about 65g) to precipitate the dye, filter cake with suction, dry the filter cake at 80~90℃ for 24h, and obtain about 116g of dye Ⅰ-2 .
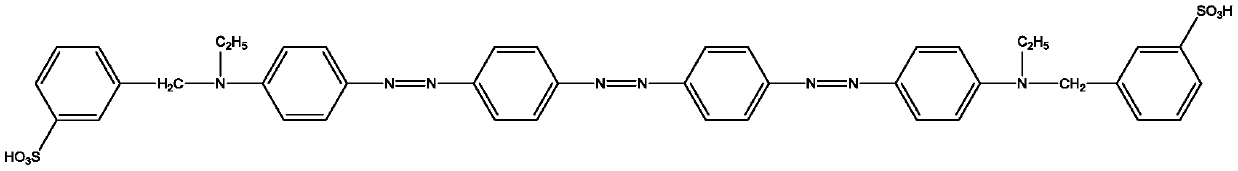
[0054] Dye Ⅰ-2 is:
[0055]
[0056] 1 H NMR (400MHz, DMSO-d6):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com