Synthesis method of 2-cyanoalkylsulfonyl 3,4-dihydronaphthalene compound
A kind of technology of cyanoalkylsulfonyl and synthesis method, applied in the field of synthesis of 3,4-dihydronaphthalene compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-26
[0040] Example 1-26 Reaction condition optimization test
[0041] With the methylene cyclopropane compound shown in formula II-1, the cyclobutanone oxime ester compound shown in formula III-1 as template, with K 2 S 2 o 5 for SO 2 Sources, the influence of different reaction conditions on the yield of the target product (I-1) was discussed, and representative examples are shown in Examples 1-26.
[0042] The reaction formula is as follows:
[0043] .
Embodiment 1
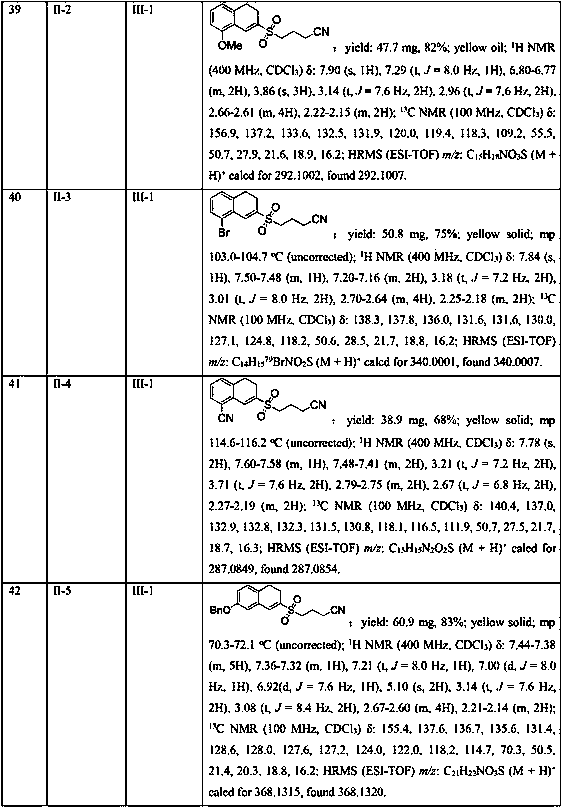
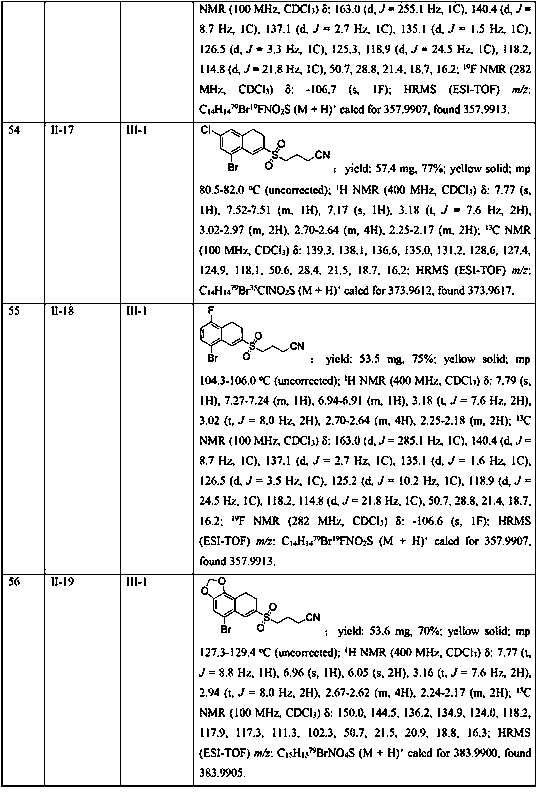
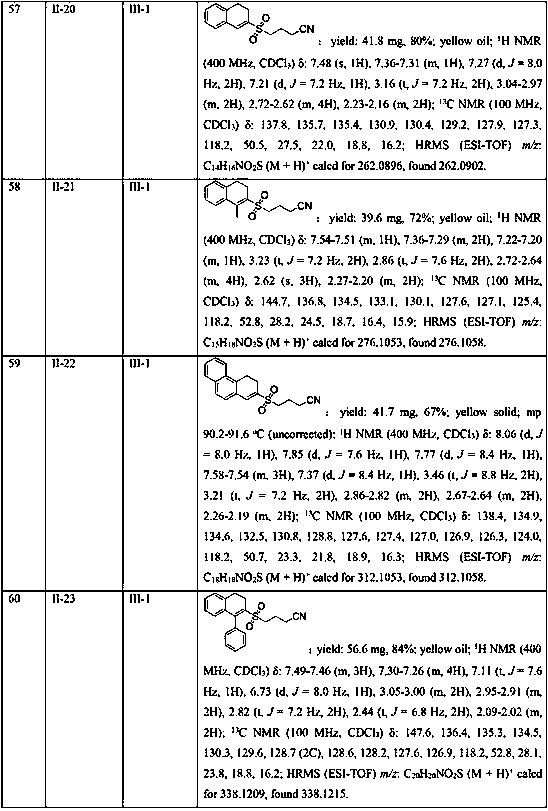
[0045] In the 25mL Schlenk lock reactor, add the methylene cyclopropane compound (0.2mmol) shown in formula II, the cyclobutanone oxime ester compound (0.3 mmol, 1.5 eqiuv) shown in formula III successively, K 2 S 2 o 5 (0.4 mmol, 2equiv), photocatalyst Ru(bpy) 3 Cl 2 (5 mol %), 2,6-lutidine (0.6 mmol, 3 eqiuv) and CH 3 CN (2 mL), then under the conditions of argon atmosphere (1atm) and 5W blue LED lamp irradiation, the oil bath was heated to 80°C and stirred for 18h. The reaction raw materials were completely consumed by TLC or GC-MS, and the reaction mixture was washed with salt Washed with water, the aqueous phase was extracted with ethyl acetate (3×10 mL), the combined phases were dried over anhydrous sodium sulfate, concentrated in vacuo to obtain a residue, and the residue was separated by silica gel column chromatography (elution solvent: n-hexane Alkane / ethyl acetate=5: 1 to 2: 1) obtain the target product shown in formula I-1. Yield: 81%. Yellow solid; mp 96.2...
Embodiment 2
[0047] Replace K with DABSO (1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct) 2 S 2 o 5 , all the other conditions and operations are the same as in Example 1, and the yield of the target product of formula I-1 is 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com