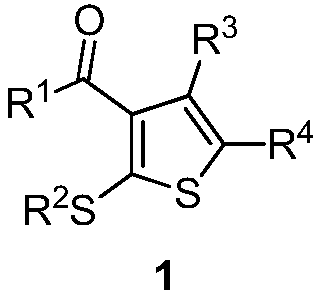
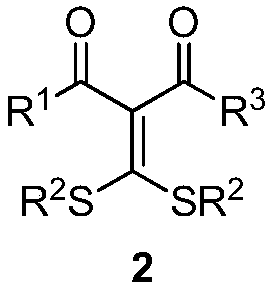
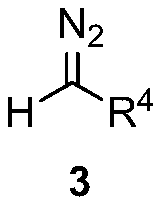
4-alkylthio polysubstituted thiophene derivative and synthesis thereof
A technology of thiophene derivatives and dialkylthiomethylene, which is applied in the field of 4-alkylthio multi-substituted thiophene derivatives, can solve problems such as violations, and achieve the effects of simple operation, low cost, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] In a 25 mL schlenk tube, add 3,3-dialkylthiomethylene-1,3-dicarbonyl compound 2a (0.5 mmol), ethyl diazoacetate 3a (1.0 mmol), ethylene iodide in sequence under argon Copper (10mol%) and 2.0 mL of 1,4-dioxane were stirred at 25°C for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=70:1) to obtain the target product 1a (101 mg, yield 78%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0036]
[0037] In a 25 mL schlenk tube, add 3,3-dialkylthiomethylene-1,3-dicarbonyl compound 2b (0.5 mmol), ethyl diazoacetate 3a (1.0 mmol), ethylene iodide in sequence under argon Copper (10mol%) and 2.0 mL of 1,4-dioxane were stirred at 25°C for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=70:1) to obtain the target product 1b (143 mg, yield 75%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0039]
[0040]The reaction steps and operations are the same as in Example 1, except that the diazonium compound is 3b. The reaction was stopped, and the target product 1c (91 mg, yield 74%) was obtained after post-processing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com