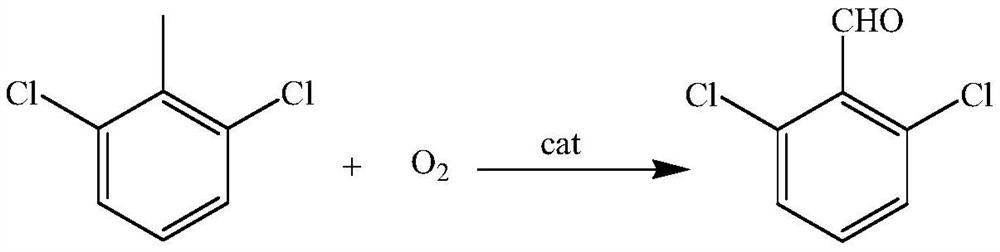A kind of synthetic method of 2,6-dichlorobenzaldehyde
A technique for the synthesis of dichlorobenzaldehyde and its synthesis method, which is applied in the field of synthesis of 2,6-dichlorobenzaldehyde, which can solve the problems that the catalyst cannot be reused, the process is long and complicated, and the sale of by-products is limited, so as to facilitate industrial production , avoid pollution, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation method of self-made composite supported catalyst:
[0029] Take 19g of copper nitrate and 29g of cobalt nitrate, add 100ml of deionized water, stir to dissolve, then add 30g of activated alumina, mix well and soak at room temperature for 12-24 hours, then dry at 110°C to constant weight, then at 600-800 calcined at ℃ for 5 to 8 hours to obtain an activated alumina-supported copper-cobalt catalyst. Weigh, and increments are calculated according to the proportion of solute in the solvent.
Embodiment 2
[0031] At room temperature, 80.5g (0.5mol) of 2,6-dichlorotoluene, 270g of methanol, 6.6g of self-made copper-cobalt composite supported catalyst (0.02mol of active ingredient) and 11g of water (0.6mol) were added to a 1000mL stainless steel autoclave. , and then closed the autoclave. Turn on the stirring and test the pressure with nitrogen until no leakage occurs.
[0032] Oxygen replaces the gas in the autoclave three times, heats up to 45°C to stop heating, controls the temperature of the autoclave to 45-60°C through the flow rate of circulating water in the jacket, uses intermittent method to introduce oxygen into the autoclave to 0.5MPa, closes the air inlet valve, when the oxygen pressure When it drops to 0.1MPa, oxygen is injected again, and the oxygen pressure is reduced from 0.5MPa to 0.1MPa for a total of 5-6 times (that is, the amount of oxygen consumed is ~0.5mol). The 2,6-dichlorotoluene ≤ 1% detected by HPLC was regarded as the end of the reaction.
[0033] The...
Embodiment 3
[0035] At room temperature, 80.5g (0.5mol) of 2,6-dichlorotoluene, 400g of methanol, 6.6g of self-made copper-cobalt composite supported catalyst (0.02mol of active ingredient) and 15g of water (0.83mol) were added to a 1000mL stainless steel autoclave. , and then closed the autoclave. Turn on the stirring and test the pressure with nitrogen until no leakage occurs.
[0036] Oxygen replaces the gas in the autoclave three times, heats up to 45°C and stops heating, controls the temperature of the autoclave to 45-55°C through the flow rate of circulating water in the jacket. When it drops to 0.1MPa, oxygen is introduced again, and the oxygen pressure is reduced from 0.5MPa to 0.1MPa for 6-7 times (ie, the amount of oxygen consumed is ~0.5mol). The 2,6-dichlorotoluene ≤ 1% in HPLC was regarded as the end of the reaction.
[0037] The reaction kettle was cooled to room temperature, and the oxygen in the kettle was evacuated. Filter the material in the kettle, rinse the filter ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com