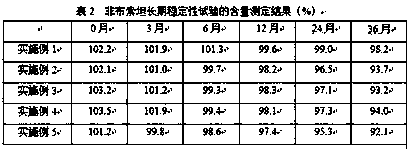
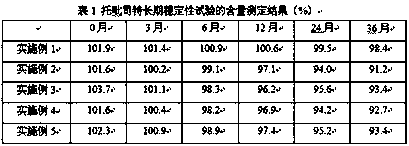
Compound controlled-release preparation containing topiroxostat
A controlled-release preparation, the technology of topiramast, is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, pharmaceutical formulas, etc., and can solve the problems of obvious side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Prescription 1 (according to 1000 capsules)
[0012] A. Topirast immediate-release granules
[0013] Topirast 5g
[0014] Microcrystalline Cellulose 2.85g
[0015] Low-substituted hydroxypropyl cellulose 6.0g
[0016] Crospovidone 0.9g
[0017] Lactose 3.0g
[0018] Magnesium stearate 0.15g
[0019] B. Topirast controlled-release granules
[0020] Topirast 15g
[0021] Microcrystalline Cellulose 8.5g
[0022] Low-substituted hydroxypropyl cellulose 7.8g
[0023] Croscarmellose 10g
[0024] Lactose 9.0g
[0025] Magnesium Stearate 4.0g
[0026] C. Febuxostat controlled-release granules
[0027] Febuxostat 40g
[0028] Hydroxypropyl Methyl Cellulose 38g
[0029] Ethylcellulose 6.0g
[0030] Lactose 6.0g
[0031] Magnesium Stearate 0.3g.
[0032] Preparation method:
[0033] (1) Topiralast and febuxostat are pulverized through an 80 mesh sieve;
[0034] (2) Mix the controlled-release material evenly, and pulverize through a 120-mesh sieve;
[0035] (3) ...
Embodiment 2
[0039] Prescription 2 (according to 1000 capsules)
[0040] A. Topirast immediate-release granules
[0041] Topirast 5g
[0042] Microcrystalline Cellulose 2.85g
[0043] Low-substituted hydroxypropyl cellulose 6.0g
[0044] Crospovidone 0.9g
[0045] Lactose 3.0g
[0046] Magnesium stearate 0.15g
[0047] B. Topirast controlled-release granules
[0048] Topirast 15g
[0049] Microcrystalline Cellulose 10.5g
[0050] Low-substituted hydroxypropyl cellulose 8.5g
[0051] Croscarmellose 12g
[0052] Lactose 8.0g
[0053] Magnesium Stearate 4.0g
[0054] C. Febuxostat controlled-release granules
[0055] Febuxostat 40g
[0056] Hydroxypropyl Methyl Cellulose 45g
[0057] Ethylcellulose 8.0g
[0058] Lactose 4.0g
[0059] Magnesium Stearate 0.3g.
[0060] Preparation method is the same as embodiment 1.
Embodiment 3
[0062] Prescription 3 (according to 1000 capsules)
[0063] A. Topirast immediate-release granules
[0064] Topirast 5g
[0065] Microcrystalline Cellulose 2.85g
[0066] Low-substituted hydroxypropyl cellulose 6.0g
[0067] Crospovidone 0.9g
[0068] Lactose 3.0g
[0069] Magnesium stearate 0.15g
[0070] B. Topirast controlled-release granules
[0071] Topirast 15g
[0072] Microcrystalline Cellulose 8.5g
[0073] Low-substituted hydroxypropyl cellulose 9.5g
[0074] Croscarmellose 10g
[0075] Lactose 9.0g
[0076] Magnesium Stearate 4.5g
[0077] C. Febuxostat controlled-release granules
[0078] Febuxostat 40g
[0079] Hydroxypropyl Methyl Cellulose 35g
[0080]Ethylcellulose 6.0g
[0081] Lactose 8.0g
[0082] Magnesium Stearate 0.25g.
[0083] Preparation method is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com